Abstract
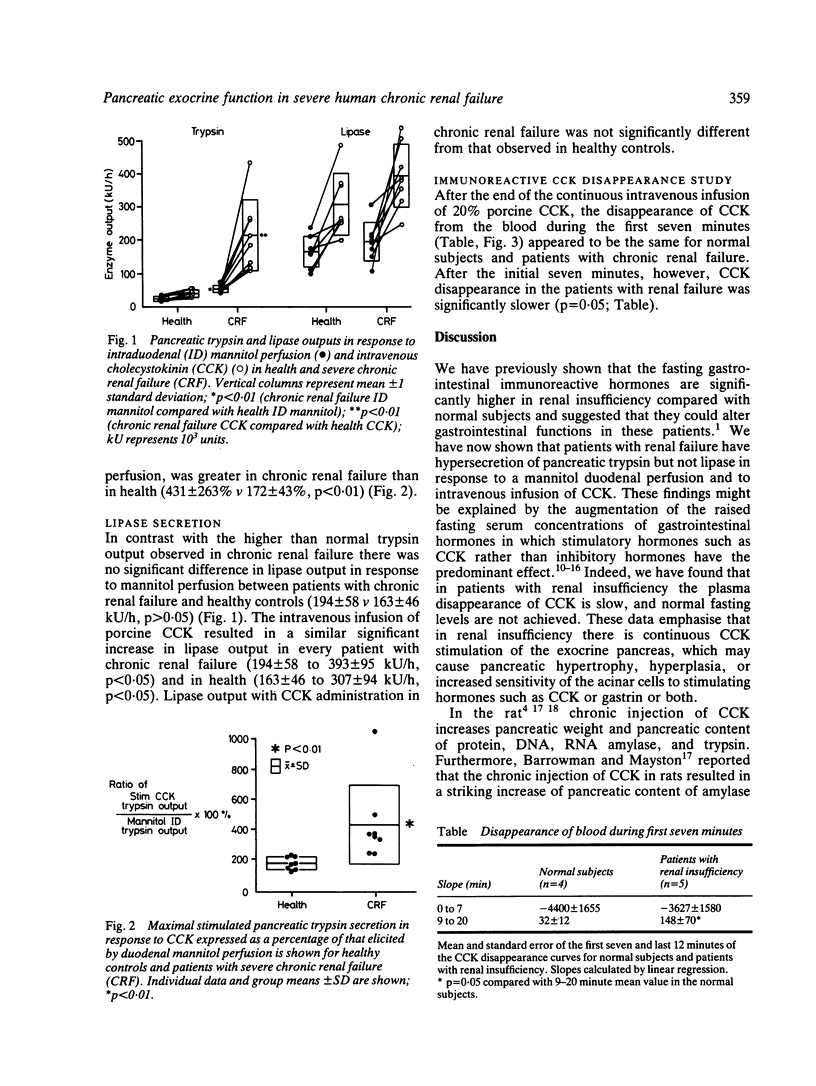
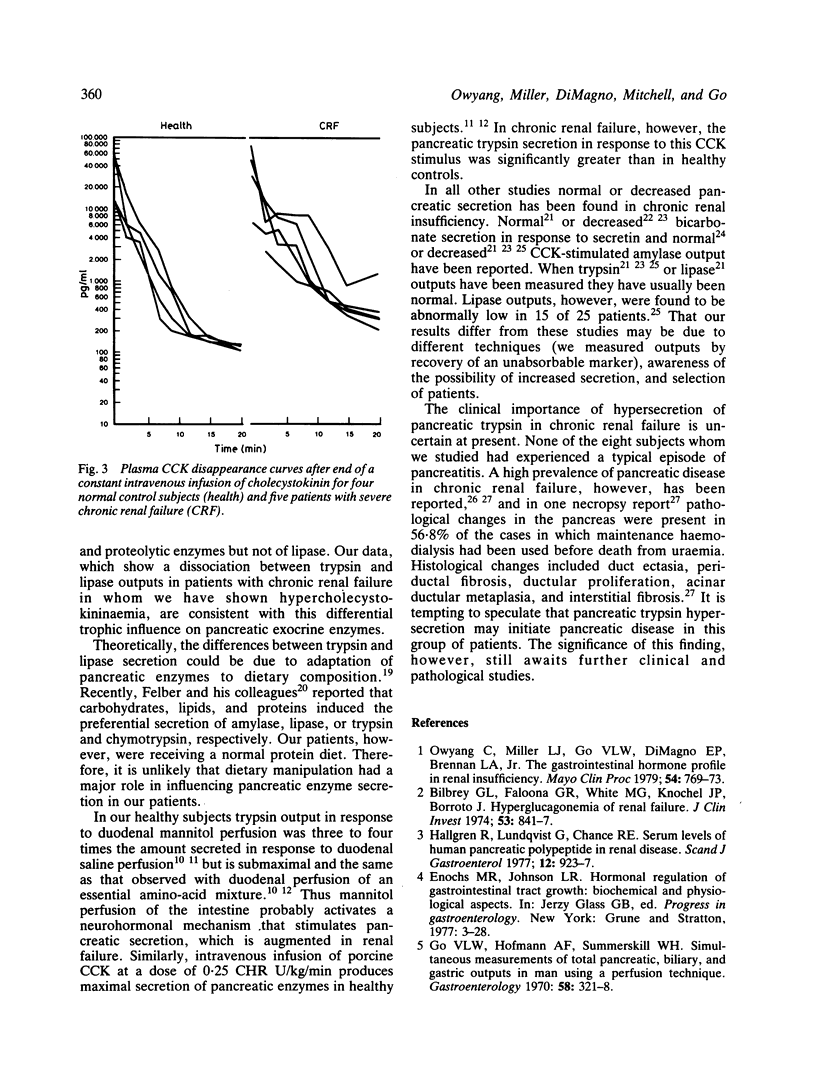
Patients with chronic renal failure have an abnormal immunoreactive gastrointestinal hormone profile, which is characterised by raised fasting serum concentrations of hormones that have antagonistic effects on exocrine pancreatic function. In addition, in this present study we have found that in renal insufficiency cholecystokinin disappears slowly from the plasma after a constant intravenous infusion of the hormone (p = 0.05 compared with healthy subjects). To evaluate whether the stimulatory or inhibitory hormones have a predominant effect, pancreatic exocrine function under conditions of mannitol perfusion of the duodenum and continuous intravenous cholecystokinin stimulation was studied in eight patients who had severe chronic renal failure and eight age-matched and sex-matched control subjects. Compared with healthy subjects, patients with renal insufficiency had hypersecretion of trypsin in response both to mannitol perfusion of the duodenum and to cholecystokinin stimulation (p less than 0.05). No significant differences in lipase secretion were noted between the patients with renal insufficiency and control subjects. These findings are consistent with the hypothesis that, of the abnormally raised fasting serum concentrations of gastrointestinal hormones found in renal insufficiency, hormones that stimulate rather than inhibit pancreatic exocrine function predominate. Secondly, the dissociation between trypsin and lipase outputs in chronic renal failure may suggest a differential trophic influence of stimulatory hormones -- that is, hypercholecystokininaemia -- on pancreatic exocrine enzyme secretion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avram M. M. High prevalence of pancreatic disease in chronic renal failure. Nephron. 1977;18(1):68–71. doi: 10.1159/000180768. [DOI] [PubMed] [Google Scholar]
- Bartos V., Melichar J., Erben J. The function of the exocrine pancreas in chronic renal disease. Digestion. 1970;3(1):33–40. doi: 10.1159/000196987. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno E. P., Go V. L., Summerskill H. J. Intraluminal and postabsorptive effects of amino acids on pancreatic enzyme secretion. J Lab Clin Med. 1973 Aug;82(2):241–248. [PubMed] [Google Scholar]
- DiMagno E. P., Go V. L., Summerskill W. H. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973 Apr 19;288(16):813–815. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Malagelada J. R., Go V. L. Relationship between alcoholism and pancreatic insufficiency. Ann N Y Acad Sci. 1975 Apr 25;252:200–207. doi: 10.1111/j.1749-6632.1975.tb19157.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt W., Stein G., Bosseckert H., Graupner C., Noske A. Exkretorische Pankreasfunktion bei Niereninsuffizienz. Z Gesamte Inn Med. 1974 Nov;29(21):895–899. [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Simultaneous measurements of total pancreatic, biliary, and gastric outputs in man using a perfusion technique. Gastroenterology. 1970 Mar;58(3):321–328. [PubMed] [Google Scholar]
- Hällgren R., Lundqvist G., Chance R. E. Serum levels of human pancreatic polypeptide in renal disease. Scand J Gastroenterol. 1977;12(8):923–927. doi: 10.3109/00365527709181351. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Obtulowicz W. Characteristics of inhibition of pancreatic secretion by glucagon. Digestion. 1974;10(2):138–149. doi: 10.1159/000197533. [DOI] [PubMed] [Google Scholar]
- Lin T. M., Evans D. C., Chance R. E., Spray G. F. Bovine pancreatic peptide: action on gastric and pancreatic secretion in dogs. Am J Physiol. 1977 Mar;232(3):E311–E315. doi: 10.1152/ajpendo.1977.232.3.E311. [DOI] [PubMed] [Google Scholar]
- Mainz D. L., Black O., Webster P. D. Hormonal control of pancreatic growth. J Clin Invest. 1973 Sep;52(9):2300–2304. doi: 10.1172/JCI107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Altered pancreatic and biliary function after vagotomy and pyloroplasty. Gastroenterology. 1974 Jan;66(1):22–27. [PubMed] [Google Scholar]
- Otte M., Stahlheber H., Forell M. M., Dobbelstein H., Richert J., Thurmayr R., Thurmayr G. R. Exokrine Pankreasfunktion bei chronischer Niereninsuffizienz. Klin Wochenschr. 1975 Jan 15;53(2):67–72. doi: 10.1007/BF01482711. [DOI] [PubMed] [Google Scholar]
- Owyang C., Miller L. J., DiMagno E. P., Brennan L. A., Jr, Go V. L. Gastrointestinal hormone profile in renal insufficiency. Mayo Clin Proc. 1979 Dec;54(12):769–773. [PubMed] [Google Scholar]
- PELOT D., GROSSMAN M. I. Distribution and fate of pancreatic enzymes in small intestine of the rat. Am J Physiol. 1962 Feb;202:285–288. doi: 10.1152/ajplegacy.1962.202.2.285. [DOI] [PubMed] [Google Scholar]
- Poll M., Werner J., Huber W., Kempmann G., Willig F. Exokrine Pankreasfunktion bei chronischer Niereninsuffizienz. Z Gastroenterol. 1979 Mar;17(3):177–186. [PubMed] [Google Scholar]
- Wittich K. A., Schmidt H., Scheler F., Creutzfeldt W. Exokrine Pankreasfunktion bei chronischer Niereninsuffizienz. Verh Dtsch Ges Inn Med. 1968;74:1072–1074. [PubMed] [Google Scholar]


