Abstract
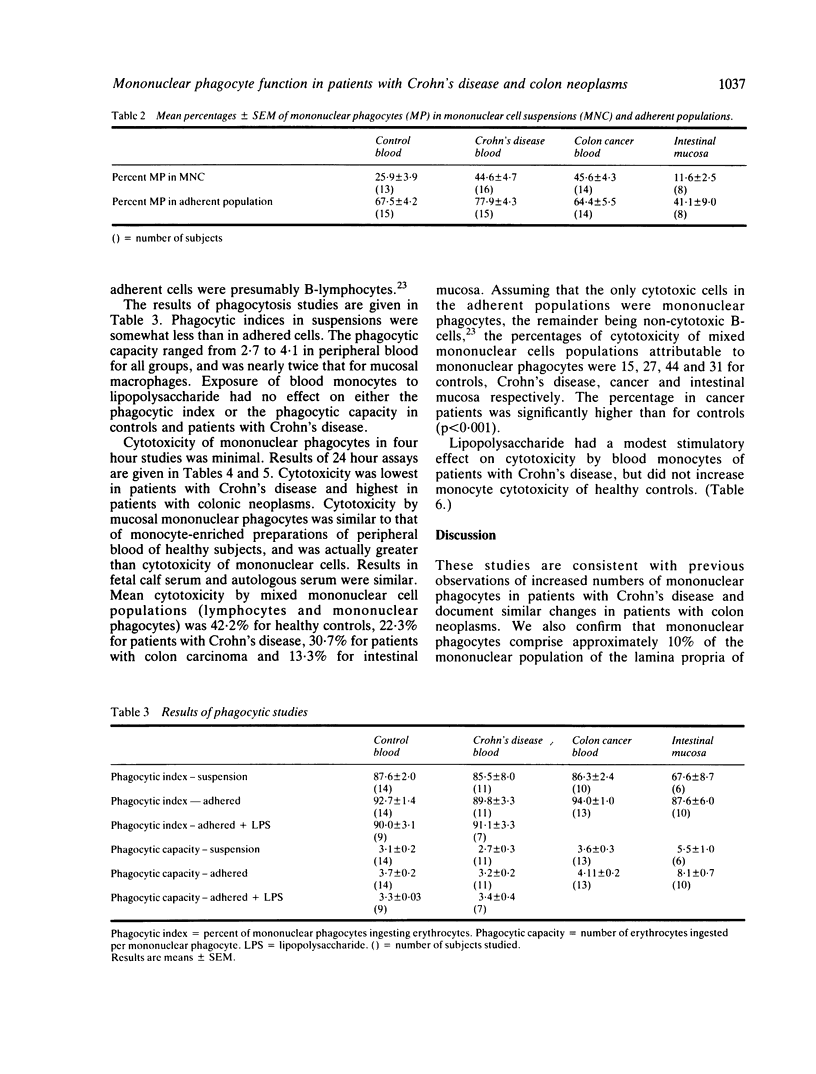
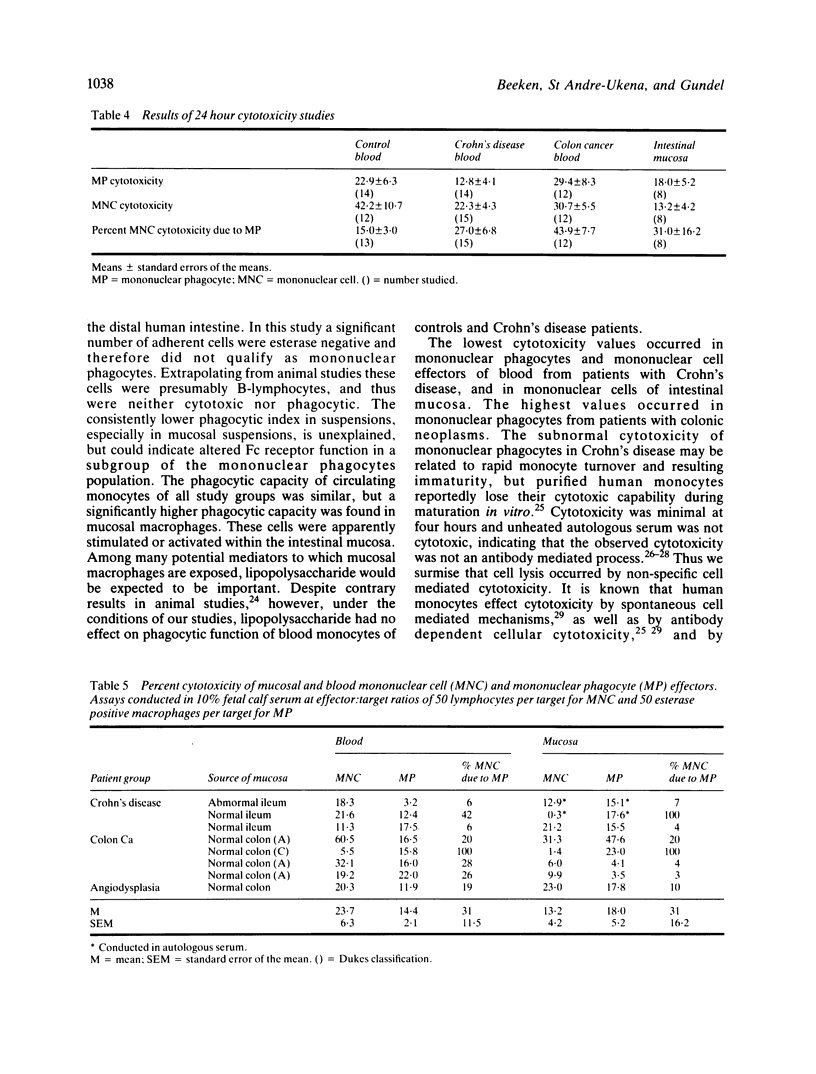
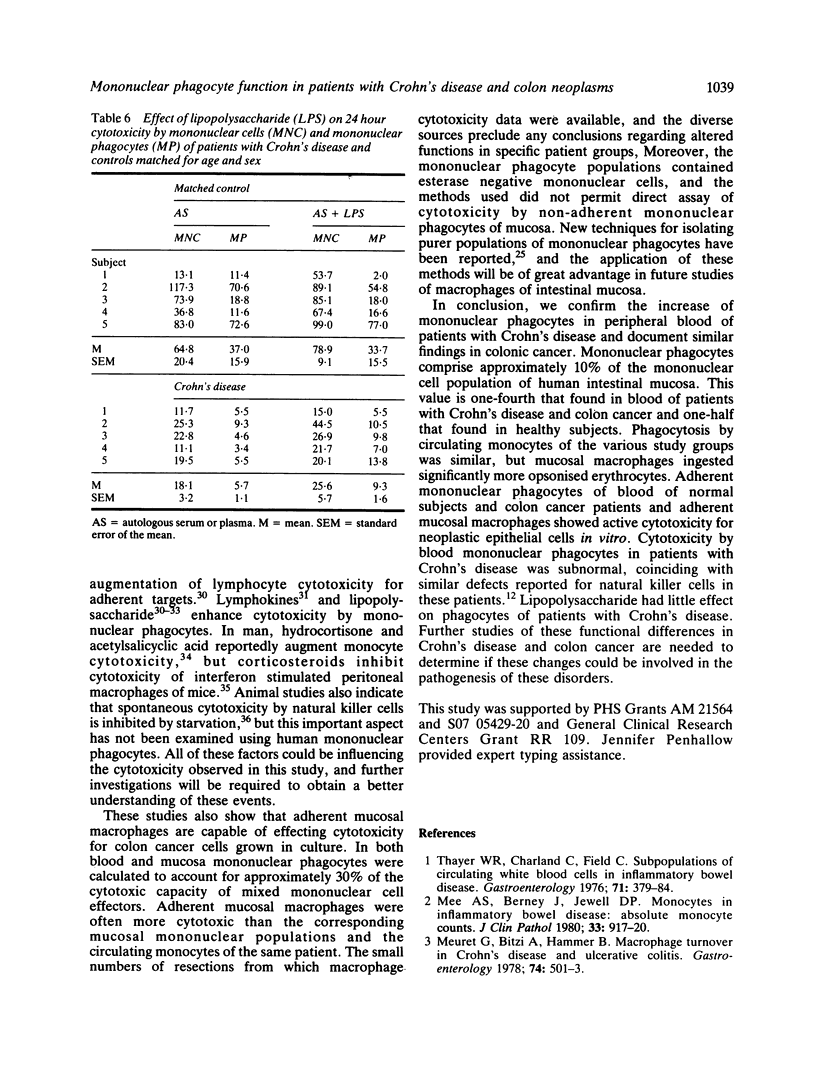
Phagocytosis and cellular cytotoxicity by mononuclear phagocytes of blood and intestinal mucosa were studied in patients with Crohn's disease and large bowel neoplasms. Antibody coated sheep erythrocytes were used for phagocytic assays and cellular cytotoxicity in vitro was measured by 24 hour isotope release from 75Selenium methionine-labelled RPMI 4788 human cancer cell cultures in the presence of mononuclear phagocyte-enriched effector populations. The mean percent of mononuclear phagocytes in Ficoll-Hypaque purified mononuclear cell suspensions of blood of healthy controls was 25.9 compared with 44.6 in patients with Crohn's disease, 45.6 in patients with colon neoplasms and 11.6 in intestinal mucosa. Phagocytic indices were similar in all groups, but the phagocytic capacity of mucosal macrophages was twice that of blood monocytes. Mean cytotoxicity of monocytes of patients with Crohn's disease was 12.8% compared with 22.9% for monocytes from normal controls, and 29.4% for patients with colon tumours. Mean cytotoxicity by mucosal macrophages was 18.0% compared with 13.2% by mucosal lymphocyte populations. Exposure of monocytes of Crohn's disease patients to bacterial lipopolysaccharide modestly increased cytotoxicity, but exposure did not alter phagocytosis by monocytes of patients or controls. The results indicate that monocytes of patients with Crohn's disease exhibit subnormal in vitro cytotoxicity. Mucosal macrophages from patients with various diseases show enhanced phagocytosis compared with blood monocytes, and they can mediate cellular cytotoxicity in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Eccles S. A., Gauci C. L. The significance of macrophages in human and experimental tumors. Ann N Y Acad Sci. 1976;276:124–133. doi: 10.1111/j.1749-6632.1976.tb41641.x. [DOI] [PubMed] [Google Scholar]
- Auer I. O., Wechsler W., Ziemer E., Malchow H., Sommer H. Immune status in Crohn's disease. I. Leukocyte and lymphocyte subpopulations in peripheral blood. Scand J Gastroenterol. 1978;13(5):561–571. doi: 10.3109/00365527809181765. [DOI] [PubMed] [Google Scholar]
- Auer I. O., Ziemer E., Sommer H. Immune status in Crohn's disease. V. Decreased in vitro natural killer cell activity in peripheral blood. Clin Exp Immunol. 1980 Oct;42(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells. Enhancement by human lymphocyte mediators. J Clin Invest. 1979 May;63(5):977–984. doi: 10.1172/JCI109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M. E., Sen L., Bachmann A. E., Pavlovsky A. Defective function of peripheral blood monocytes in patients with Hodgkin's and non-Hodgkin's lymphomas. Cancer. 1980 Jul 15;46(2):299–302. doi: 10.1002/1097-0142(19800715)46:2<299::aid-cncr2820460214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ganguly N. K., Kingham J. G., Lloyd B., Lloyd R. S., Price C. P., Triger D. R., Wright R. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet. 1978 May 20;1(8073):1073–1075. doi: 10.1016/s0140-6736(78)90917-0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Chapman H. A., Jr, Weinberg J. B. The macrophage as an antineoplastic surveillance cell: biological perspectives. J Reticuloendothel Soc. 1978 Nov;24(5):549–570. [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Louie J. S., Wahl L. M., Muchmore A. V. Pharmacology of human spontaneous monocyte-mediated cytotoxicity. I. Enhancement by salicylates and steroids. Arthritis Rheum. 1981 Jun;24(6):774–780. doi: 10.1002/art.1780240604. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Crosby W. H. Histochemical characterization of cellular and structural elements of the human spleen. J Histochem Cytochem. 1972 Dec;20(12):1049–1058. doi: 10.1177/20.12.1049. [DOI] [PubMed] [Google Scholar]
- Mee A. S., Berney J., Jewell D. P. Monocytes in inflammatory bowel disease: absolute monocyte counts. J Clin Pathol. 1980 Oct;33(10):917–920. doi: 10.1136/jcp.33.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee A. S., Jewell D. P. Monocytes in inflammatory bowel disease: monocyte and serum lysosomal enzyme activity. Clin Sci (Lond) 1980 Apr;58(4):295–300. doi: 10.1042/cs0580295. [DOI] [PubMed] [Google Scholar]
- Mee A. S., Szawatakowski M., Jewell D. P. Monocytes in inflammatory bowel disease: phagocytosis and intracellular killing. J Clin Pathol. 1980 Oct;33(10):921–925. doi: 10.1136/jcp.33.10.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret G., Bitzi A., Hammer B. Macrophage turnover in Crohn's disease and ulcerative colitis. Gastroenterology. 1978 Mar;74(3):501–503. [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- Nelson D. L., Bundy B. M., Strober W. Spontaneous cell-mediated cytotoxicity by human peripheral blood lymphocytes in vitro. J Immunol. 1977 Oct;119(4):1401–1405. [PubMed] [Google Scholar]
- Russell J. H., Masakowski V. R., Dobos C. B. Mechanisms of immune lysis. I. Physiological distinction between target cell death mediated by cytotoxic T lymphocytes and antibody plus complement. J Immunol. 1980 Mar;124(3):1100–1105. [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A. The mechanism of K cell (antibody-dependent) cell mediated cytotoxicity. I. The release of different cell components. Proc R Soc Lond B Biol Sci. 1977 Jul 20;197(1129):407–415. doi: 10.1098/rspb.1977.0077. [DOI] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Regulation of natural killer activity in vivo: Part I--loss of natural killer activity during starvation. Indian J Exp Biol. 1980 Dec;18(12):1383–1386. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Monocyte IgG receptor activity, dynamics, and modulation-normal individuals and patients with granulomatous diseases. J Lab Clin Med. 1977 Feb;89(2):332–340. [PubMed] [Google Scholar]
- Schubert R. D., David J. R. Stimulation of guinea pig macrophage pinocytosis by lipopolysaccharides (LPS): evidence that LPS acts directly on the macrophage. Cell Immunol. 1980 Sep 15;55(1):166–173. doi: 10.1016/0008-8749(80)90147-1. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A., Stoychkov J. N., Pavlidis N. A. Factors affecting macrophage cytotoxic activity with particular emphasis on corticosteroids and acute stress. J Reticuloendothel Soc. 1979 Jul;26(1):83–92. [PubMed] [Google Scholar]
- Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981 Sep;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Taramelli D., Holden H. T., Varesio L. In vitro induction of tumoricidal and suppressor macrophages by lymphokines: possible feedback regulation. J Immunol. 1981 Jun;126(6):2123–2128. [PubMed] [Google Scholar]
- Thayer W. R., Jr, Charland C., Field C. E. The subpopulations of circulating white blood cells in inflammatory bowel disease. Gastroenterology. 1976 Sep;71(3):379–384. [PubMed] [Google Scholar]
- Whorwell P. J., Bennett P., Tanner A. R., Wright R. Monocyte function in Crohn's disease and ulcerative colitis. Digestion. 1981;22(5):271–275. doi: 10.1159/000198668. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. Detection and quantitation of macrophage infiltration into primary human tumors with the use of cell-surface markers. J Natl Cancer Inst. 1977 Oct;59(4):1081–1087. doi: 10.1093/jnci/59.4.1081. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Neff J. R. A reevaluation of B-lymphocyte levels in peripheral blood from cancer patients. J Natl Cancer Inst. 1978 Sep;61(3):715–718. [PubMed] [Google Scholar]
- Woodward J. G., Daynes R. A. Cell-mediated immune response to syngeneic UV induced tumors. I. The presence of tumor associated macrophages and their possible role in the in vitro generation of cytotoxic lymphocytes. Cell Immunol. 1978 Dec;41(2):304–319. doi: 10.1016/0008-8749(78)90228-9. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Mendelsohn J., Bont W. S. Requirement for monocytes in the spontaneous cytotoxic effects of human lymphocytes against non-lymphoid target cells. Nature. 1980 Feb 7;283(5747):574–576. doi: 10.1038/283574a0. [DOI] [PubMed] [Google Scholar]


