Abstract
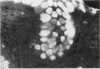
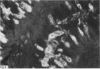
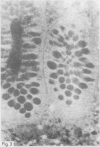
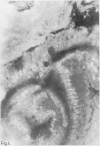
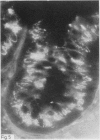
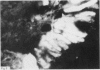
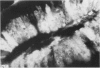
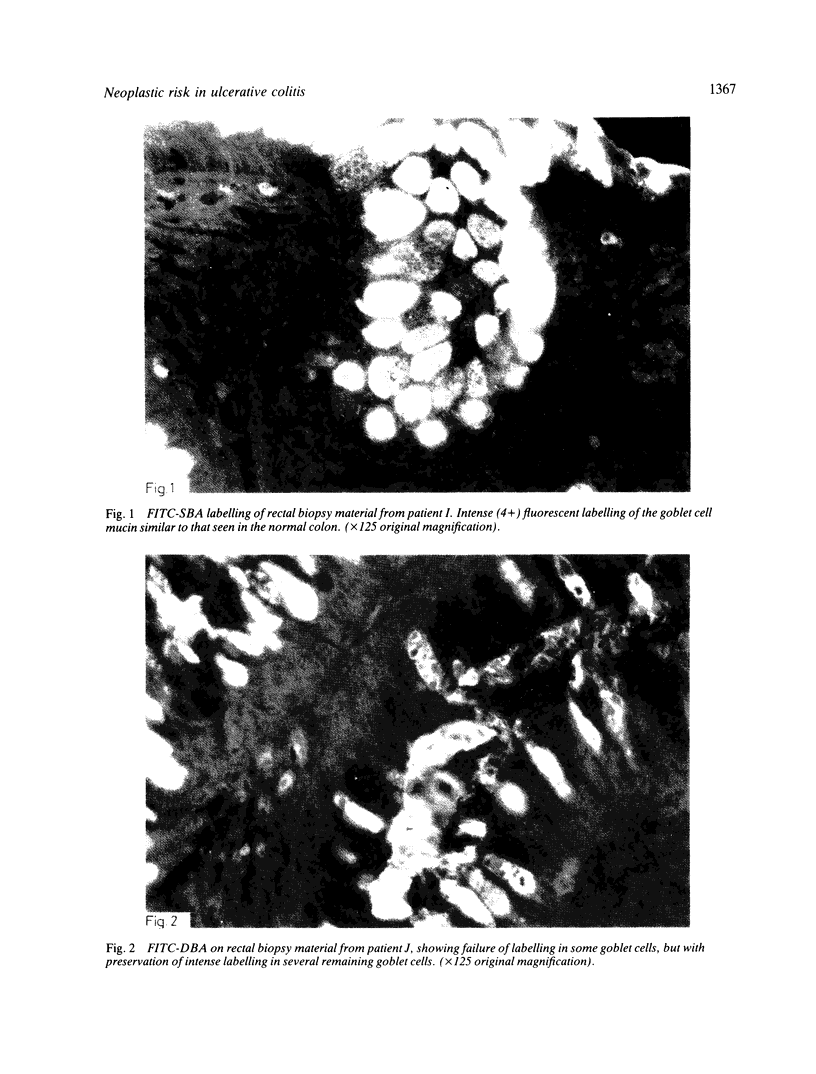
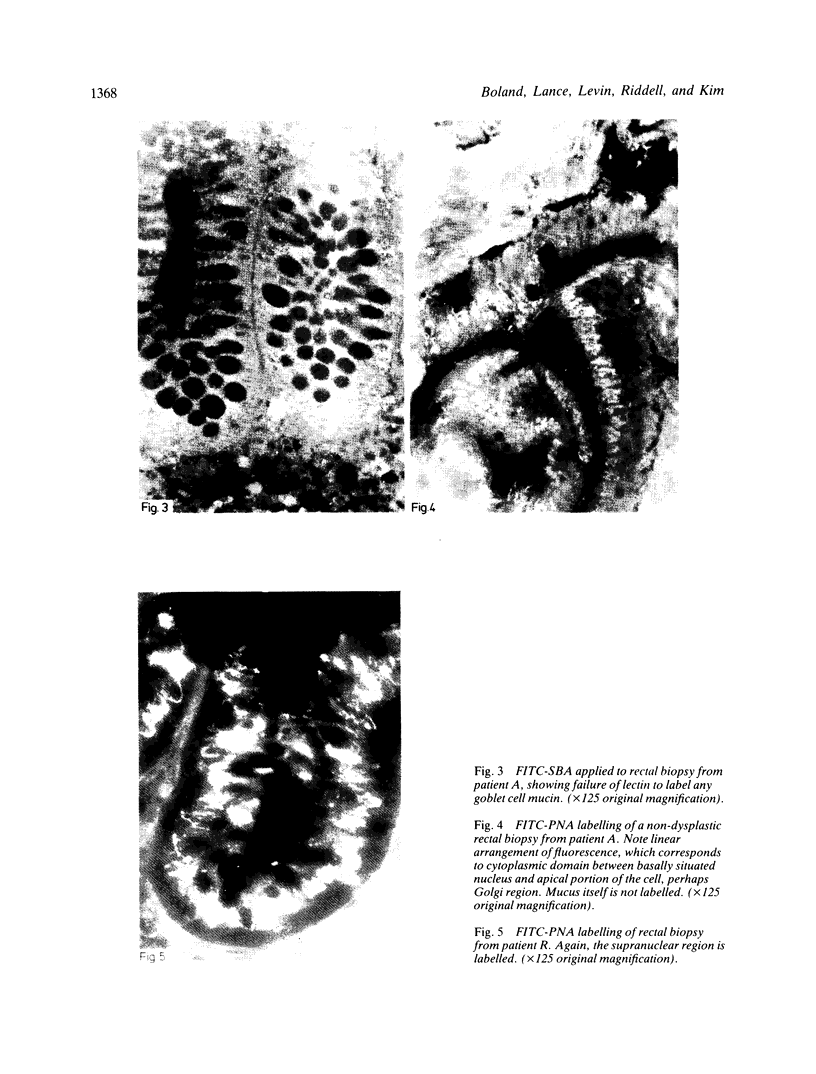
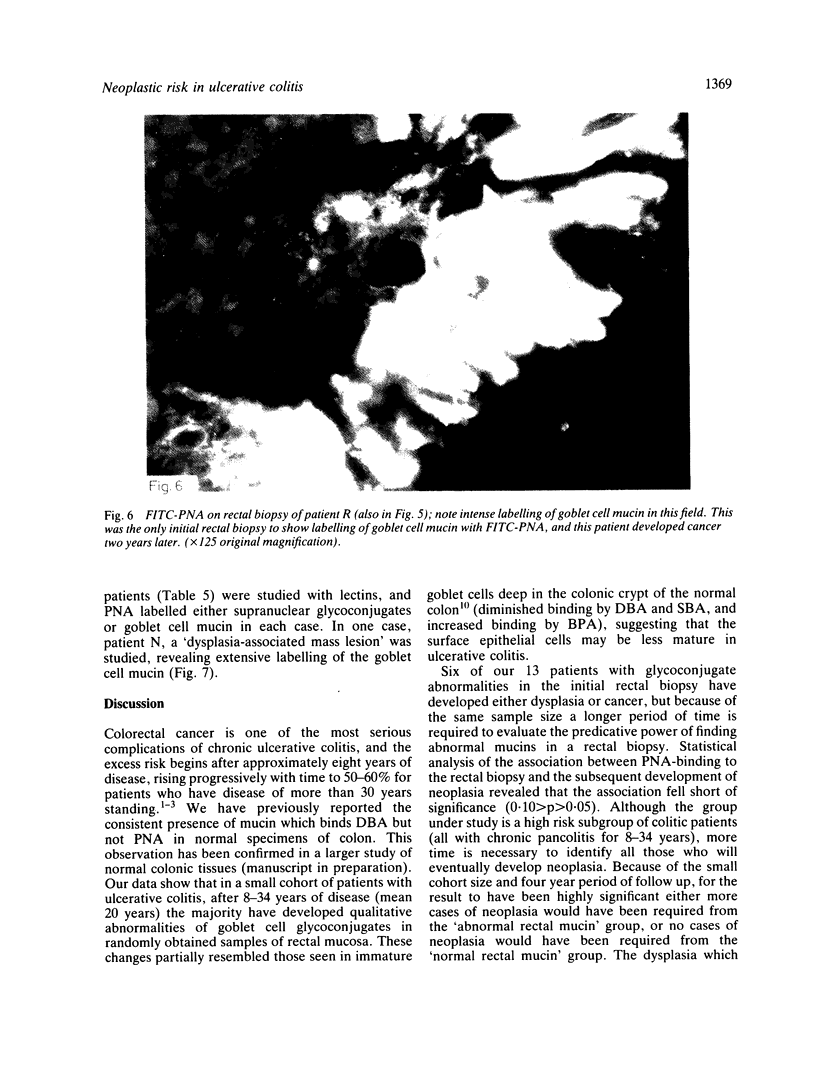
A group of 18 patients with stable ulcerative colitis involving the entire colon for at least eight years was subjected to a biopsy of normal appearing rectal mucosa and followed prospectively over four years for the development of either dysplasia or cancer. Goblet cell glycoconjugate structure was examined in the rectal biopsies using fluorescein conjugated lectins. At the beginning of the study, 13 of the 18 patients had abnormalities of goblet cell mucin or cytoplasmic glycoconjugates in the rectal biopsies. Dysplasia subsequently developed in six and carcinoma in one of these patients. Among the five patients with normal lectin binding studies in the initial rectal biopsies, colonic dysplasia has subsequently developed in one. The abnormalities seen in the rectal goblet cells resembled in part those previously seen in immature and neoplastic colonic cells. The dysplastic tissues all contained the form of mucin which has been found in other neoplastic colonic tissues. This preliminary report after four years of prospective study suggests that abnormalities of glycoconjugate structure may be associated with, and may precede, neoplastic events in the setting of chronic ulcerative colitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland C. R., Montgomery C. K., Kim Y. S. A cancer-associated mucin alteration in benign colonic polyps. Gastroenterology. 1982 Apr;82(4):664–672. [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt J. H., Lennard-Jones J. E. A practical approach to the risk of cancer in inflammatory bowel disease. Med Clin North Am. 1980 Nov;64(6):1203–1220. doi: 10.1016/s0025-7125(16)31564-4. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Fraser G., Read A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981 Aug;61(2):229–234. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- Devroede G. J., Taylor W. F., Sauer W. G., Jackman R. J., Stickler G. B. Cancer risk and life expectancy of children with ulcerative colitis. N Engl J Med. 1971 Jul 1;285(1):17–21. doi: 10.1056/NEJM197107012850103. [DOI] [PubMed] [Google Scholar]
- EDWARDS F. C., TRUELOVE S. C. THE COURSE AND PROGNOSIS OF ULCERATIVE COLITIS. III. COMPLICATIONS. Gut. 1964 Feb;5:1–22. doi: 10.1136/gut.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Morphological and mucus secretion criteria for differential diagnosis of solitary ulcer syndrome and non-specific proctitis. J Clin Pathol. 1982 Jan;35(1):26–30. doi: 10.1136/jcp.35.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Mucin secretion in inflammatory bowel disease: correlation with disease activity and dysplasia. Gut. 1982 Jun;23(6):485–489. doi: 10.1136/gut.23.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Dawson I. The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn's disease. Gut. 1970 Mar;11(3):229–234. doi: 10.1136/gut.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Mughal S., Bussey H. J. Patterns of mucus secretion in the colonic epithelium in familial polyposis. Invest Cell Pathol. 1980 Oct-Dec;3(4):329–343. [PubMed] [Google Scholar]
- Greco V., Lauro G., Fabbrini A., Torsoli A. Histochemistry of the colonic epithelial mucins in normal subjects and in patients with ulcerative colitis. A qualitative and histophotometric investigation. Gut. 1967 Oct;8(5):491–496. doi: 10.1136/gut.8.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein A. J., Sachar D. B., Smith H., Pucillo A., Papatestas A. E., Kreel I., Geller S. A., Janowitz H. D., Aufses A. H., Jr Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology. 1979 Aug;77(2):290–294. [PubMed] [Google Scholar]
- Hellstrom H. R., Fisher E. R. Estimation of mucosal mucin as an aid in the differentiation of Crohn's disease of the colon and chronic ulcerative colitis. Am J Clin Pathol. 1967 Sep;48(3):259–268. doi: 10.1093/ajcp/48.3.259. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Attwood P. R. Failure to demonstrate specificity of the morphological and histochemical changes in mucosa adjacent to colonic carcinoma (transitional mucosa). J Clin Pathol. 1979 Mar;32(3):214–218. doi: 10.1136/jcp.32.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Isaacs R., Perdomo J. M. Alterations of membrane glycopeptides in human colonic adenocarcinoma. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4869–4873. doi: 10.1073/pnas.71.12.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]