Abstract
Little is known about the functional connectivity between astrocytes in the CNS. To explore this issue we photo-released glutamate onto a single astrocyte in murine hippocampal slices and imaged calcium responses. Photo-release of glutamate causes a metabotropic glutamate receptor (mGluR)-dependent increase in internal calcium in the stimulated astrocyte and delayed calcium elevations in neighboring cells. The delayed elevation in calcium was not caused by either neuronal activity following synaptic transmission or by glutamate released from astrocytes. However, it was reduced by flufenamic acid (FFA), which is consistent with a role for adenosine triphosphate (ATP) release from astrocytes as an intercellular messenger. Exogenous ligands such as ATP (1 μM) increased the number of astrocytes that were recruited into coupled astrocytic networks, indicating that extracellular accumulation of neurotransmitters modulates neuronal excitability, synaptic transmission and functional coupling between astrocytes.
Keywords: Fluo-4, GFAP, photolysis, caged glutamate, glia, calcium
INTRODUCTION
An increasing body of evidence has shown that neurons and astrocytes are bidirectionally connected through chemical transmitter signaling pathways (Pasti et al., 1997; Araque et al., 1998a; Araque et al., 1998b; Kang et al., 1998; Newman, 2003). Neuronal activity signals to postsynaptic neurons and to synaptically-associated astrocytes by acting on metabotropic receptors on astrocytes (Porter and McCarthy, 1996). Activation of these receptors leads to a calcium elevation in the astrocyte which can, in turn, cause the release of chemical transmitters including glutamate (Parpura et al., 1994; Parpura and Haydon, 2000), ATP (Guthrie et al., 1999; Newman, 2001; Wang et al., 2000) and prostaglandin E2 (Bezzi et al., 1998). As a consequence, transmitters released from astrocytes regulate neuronal activity and synaptic transmission (Araque et al., 1999; Haydon, 2001; Volterra et al., 2002).
Whereas neurons rely on voltage-related signals to propagate information over distances, astrocytes, which are usually electrically silent, use slower conducting calcium oscillations as their form of signal propagation (Verkhratsky and Kettenmann, 1996). Although individual astrocytes can exhibit calcium oscillations in brain-slice preparations (Nett et al., 2002), there is little information regarding the spread of calcium signals between astrocytes in vivo. Extensive studies performed using cells in culture (Charles et al., 1991; Cotrina et al., 1998; Guthrie et al., 1999; Wang et al., 2000) and organotypic slice cultures (Harris-White et al., 1998) demonstrate that stimuli that elevate astrocytic calcium evoke long-range propagating waves between adjacent astrocytes. However, it is likely that the properties of astrocytes change during culture, so it is not known whether these long-range calcium waves occur in the intact nervous system.
To address this issue, acutely isolated tissue has been used to study calcium signaling. Electrical stimulation in brain slices initiates long-range, ATP-dependent calcium waves between glia (Schipke et al., 2002). Additionally, mechanical stimulation of individual glial cells in the retina leads to ATP-dependent propagation of calcium waves between astrocytes and Muller glial cells. However, whether physiological signals, such as neurotransmitters, induce long-range calcium waves between astrocytes is unclear. To address this issue, we used photolysis to focally administer glutamate to individual astrocytes in acutely isolated hippocampal-slice preparations. We show that this stimulus elevates calcium in the stimulated cell and causes delayed calcium responses in its immediate neighbors.
OBJECTIVE
The objective of this study was to determine whether astrocytes interconnect with one another in CNS tissue. To achieve this, we loaded acutely isolated hippocampal-slice preparations with calcium indicators, and focally applied the neurotransmitters glutamate and bradykinin by UV-mediated photolysis while performing confocal calcium imaging.
METHODS
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Swiss-Webster mice (Charles River Laboratories) and pGFAP/GFP mice (Zhuo et al., 1997) (Jackson Laboratories), 7–12-days old, were anesthetized with halothane, decapitated and their brains placed in ice-cold (3–4°C) artificial cerebrospinal fluid (aCSF; NaCl 124 mM, KCl 3.1 mM, either MgSO4 1 mM or MgCl2 1 mM, CaCl2 2 mM, NaH2PO4 1.25 mM, glucose 10 mM, NaHCO3 26 mM). Horizontal slices (250 μm thick) were cut in aCSF gassed with 5% CO2:95% O2 using a VT2000 (Leica). After a 1-hr recovery period, the slices were either transferred to a calcium indicator loading chamber or maintained in a holding chamber until needed. Slices were loaded with calcium indicator by incubation in freshly prepared (12.5 μg ml−1) X-rhod-1 or fluo-4 AM for 60–90 mins at room temperature. Slices were then transferred to a recording chamber for microscopy.
In initial studies we used pGFAP/GFP mice and the calcium indicator X-rhod-1 to positively identify astrocytes in brain slices using GFP fluorescence. Bulk-loading of brain slices with AM indicators preferentially loads non-neuronal cells, and 97% of X-rhod-1-labeled cells were GFP-positive. Subsequently, we used fluo-4 AM where possible, because of its greater dynamic range, and used brain slices from Swiss-Webster mice. Although we cannot discount the possibility that fluo-4 AM loads additional cell types, such as microglia, similar data were obtained with both strains of mice and both indicators.
Imaging was performed initially using an Odyssey confocal microscope (Noran, Middleton, WI) attached to an Olympus BX50 fixed-stage upright microscope, with excitation at 564 nm and 488 nm for imaging GFP and X-rhod-1, respectively. Subsequently, fluo-4 imaging was performed using a confocal scan head (Prairie Technologies, Middleton, WI) attached to an Olympus BX51 fixed-stage upright microscope. Both systems use an Olympus 40x water-immersion objective (N.A. 0.8). In all studies, 30 images were collected at 3-second intervals. Changes in fluorescence are reported as background-subtracted ΔF/Fo. Images were obtained from astrocytes in stratum radiatum in area CA1 of the hippocampus. Experiments were carried out at room temperature (20–23°C).
To provide focal stimuli to individual astrocytes, we photo-released glutamate from γ-(CNB-caged) l-glutamic acid using a photolysis head (Prairie Technologies) placed between the confocal microscope and the fixed-stage microscope. One of two photolysis systems used: either a twin-epi photolysis system coupled to a nitrogen-pulsed laser (337 nm) or a single-path photolysis head connected to an Enterprise continuous wave argon laser (351 nm and 364 nm) (Coherent Technologies, Santa Clara, CA). In these systems the photolysis spot (~3 μm diameter) was moved within the image using remote-controlled stepper motors. A red laser (635 nm) was used to locate the beam and assist positioning. Photolysis was performed using pulses of 1–20 ms with 0.6–1.3 mW power at the back focal plane of the objective.
Reagents were purchased from the following vendors: ATP, FFA acetylcholine (ACh) and bafilomycin A1 (Sigma, St Louis, MO); d-2-amino-5-phosphonopentanoic acid (D-AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and (S)-α-methyl-4-carboxyphenylglycine (MCPG) 2-aminoethoxydiphenyl borate (2-APB); cyclopiazonic acid (CPA) (Tocris, Ellisville, MO); tetrodotoxin (TTX), U-73122 and U-73343 (Calbiochem, La Jolla, CA); fluo-4 AM, X-rhod-1 AM and γ-(CNB-caged) l-glutamic acid (Molecular Probes, Eugene, OR). All drugs were made as stock solutions and diluted to their final concentration in bathing medium before use. Student’s t-test was performed to determine statistical significance. Data are expressed as mean ± sem.
RESULTS
To determine whether astrocytes interconnect functionally, we used photolysis to provide a spatio–temporally controlled stimulus similar to that provided by synaptic inputs. Initially, we used pGFAP/GFP mice (Zhuo et al., 1997) in which astrocytes express GFP (Fig. 1A). Loading acutely isolated brain slices with the red-shifted calcium indicator X-rhod-1 allowed us to measure the relative change in calcium in identified astrocytes (Fig. 1B). Photo-release of glutamate from γ-(CNB-caged) l-glutamic acid (200 μM) onto the cell bodies of individual astrocytes evoked an increase in calcium in the stimulated cell. This was caused by activation of mGluRs because incubation with the mGluR antagonist MCPG (20 μM) prevented the increase in calcium. UV stimulation in the absence of γ-(CNB-caged) l-glutamic acid confirmed that the response was caused by photo-liberation of glutamate rather than by UV illumination directly (Fig. 2). In addition, neither TTX (10 μM) nor bafilomycin A1 (2.5 μM), which block neuronal activity and synaptic transmission, respectively, affect the calcium response of stimulated astrocytes (Fig. 2), which indicates that this response is brought about by photo-released glutamate. Consistent with activation of mGluRs, the calcium elevation is prevented by U-73122 (20 μM), a phospholipase C (PLC) antagonist, but not by its inactive analog U-73343 (20 μM), by the inositol (1,4,5)-trisphosphate (IP3) receptor antagonist 2-APB (200 μM) (Maruyama et al., 1997) and by depletion of internal calcium stores using CPA (40 μM) (Fig. 3).
Fig. 1. Photo-release of glutamate elicits calcium signals in astrocytes.
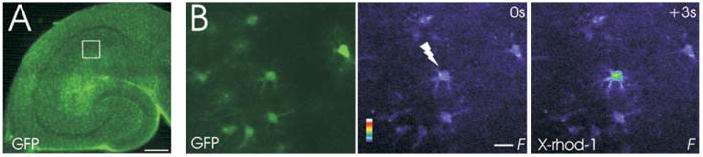
(A) Fluorescent image of hippocampal brain slice from a pGFAP/GFP transgenic mouse that was used to identify astrocytes. Astrocytes in brain slices express GFP. The square represents a typical area in which astrocytes were imaged within the stratum radiatum of CA1. (B) GFP fluorescence (left) was used to identify X-rhod-1-loaded astrocytes (center). Flash photolysis (337 nm) of caged glutamate (200 μM) elicited a change in calcium concentration in the directly stimulated astrocyte (right). Color scale represents the linear pseudocolor scale (0–255). Scale bars: A, 200 μm; B, 20 μm.
Fig. 2. Photo-released glutamate stimulates a mGluR-dependent calcium increase in astrocytes.
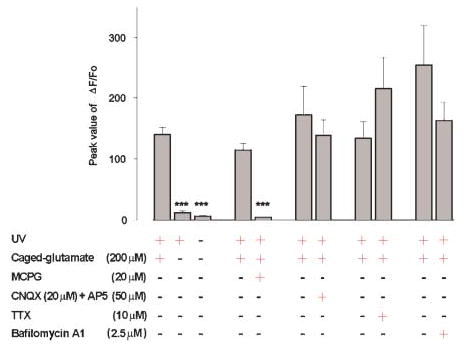
The peak change in fluorescence intensity of X-rhod-1 in response to photolysis is shown. The increase in calcium in the stimulated cell (n=72) is caused by photo-release of glutamate because omission of caged glutamate (n=56) prevented UV-induced changes in calcium, and because MCPG (n=9), but not CNQX (n=11) and D-AP5 (n=11), blocks the glutamate-induced calcium increase. Photolysis-induced calcium changes do not require neuronal activity or synaptic transmission because application of TTX (n=12) and bafilomycin A1 (n=16) do not reduce the magnitude of the astrocytic calcium response. *** P<0.001.
Fig. 3. Photo-release of glutamate induces mGluR-dependent release of calcium from IP3-sensitive internal stores.
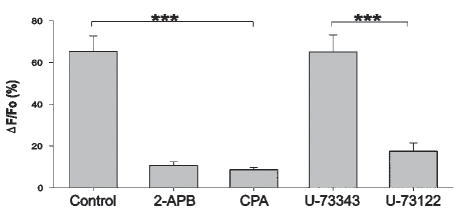
Photolysis-induced calcium elevation is reduced by the IP3 receptor antagonist 2-APB (n=17, control n=11) and by depletion of internal calcium stores following incubation in the calcium-ATPase inhibitor CPA (n=12). The specific PLC inhibitor U-73122 reduces the glutamate-induced increase in calcium (n=14) comparison with the inactive analog U-73343 (n=5) *** P<0.001.
Following direct stimulation of a single astrocyte, we found that neighboring astrocytes have a delayed elevation in calcium concentrations. Figure 4 shows the calcium responses of four astrocytes following a single stimulus to one cell. Repeated stimulation of the same cell caused similar delayed responses in its neighbors (Fig. 4A,B). On average, the first stimulus evoked calcium responses in 3.3 ± 1.1 cells, and the second evoked responses in 4.2 ± 0.9 cells (n=5) (Fig. 4C). Of the cells that responded to the first stimulus, 77.6 ± 9.8 % responded to a second. However, not all adjacent astrocytes are recruited into this connectivity. For example, Fig. 4C depicts all the astrocytes in the field of view, determined by the ability to respond with a calcium elevation following perfusion with ATP (100 μM). Cells colored black did not exhibit calcium responses following photo-stimulation of the primary astrocyte. Note that the arrow identifies unconnected cells between the stimulated cell (lightning bolt) and a more distant, connected cell (*). Clearly this unresponsive cell is functional because ATP evokes a calcium elevation. We frequently observed cells that were not connected functionally in circuits but that were responsive to ATP. We photo-released glutamate onto four of these cells and demonstrated that each was connected functionally to other astrocytes. This indicates that there are distinct functional circuits between astrocytes in the hippocampus.
Fig. 4. Astrocytes are interconnected in short-range circuits.
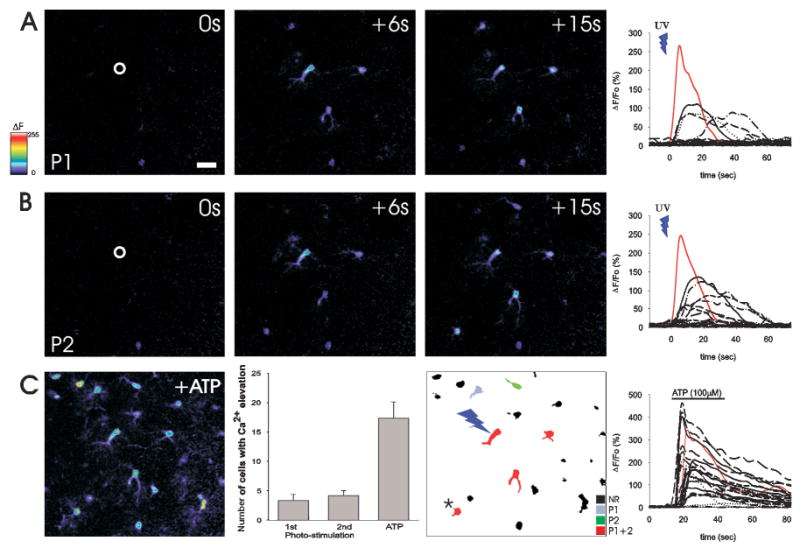
(A) Photo-release of glutamate (P1, in position noted by circle) causes a rapid increase in calcium in the stimulated astrocyte (red trace in graphs) and delayed calcium responses in neighboring astrocytes (black trace), indicating the presence of connectivity between these glial cells. (B) A second stimulus to the same astrocyte (P2) demonstrates that many responsive astrocytes are connected reliably to the stimulated cell. (C) Panel 1: Addition of ATP (100 μM) reveals the total number of glial cells in the imaging field. Panel 2: The number of astrocytes in one optical section that respond to photolytic stimulation of a primary astrocyte. Note that there is no difference in the number of responsive astrocytes when two sequential photo-stimuli are provided, and that a small proportion of the total number of cells in the imaging field (ATP-responsive cells) are connected functionally to the stimulated astrocyte. Panel 3: The positions of ATP-responsive cells. Red cells represent those that respond to both photolysis stimuli (P1+P2) whereas some cells are connected less reliably (P1 or P2 alone). Black cells represent ATP-responsive cells that are not functionally connected to the astrocyte that was stimulated by photo-release of glutamate. An unconnected cell (arrow) is located between the stimulated cell (lightning bolt) and a more distant connected astrocyte (*), which indicates that connectivity is specific. Color scale represents the linear pseudocolor scale (0–255). Scale bar: 20 μm.
To investigate whether indirect astrocytic responses to photolysis were caused by the diffusion of photo-released glutamate to neighboring cells, we asked whether photolysis of caged glutamate adjacent to the cell body of an astrocyte evoked a calcium response. Although photolysis on the cell body reliably evoked an astrocytic calcium response, this response did not occur when the point of photolysis was displaced laterally by a distance of 33.3 ± 2.1 μm (n=7) (Fig. 5). Thus, diffusion of glutamate from the point of photolysis to adjacent astrocyte somata does not account for the ability of a stimulated cell to recruit additional astrocytes into a functional circuit, which suggests that these glial cells are functionally interconnected.
Fig. 5. Photo-released glutamate does not diffuse to stimulate distant astrocytes.
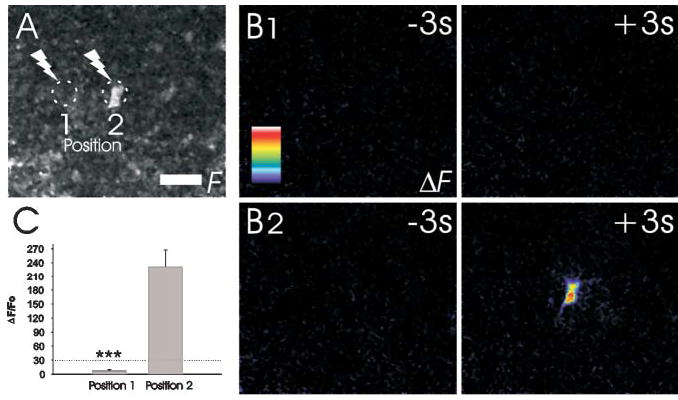
(A) Monochrome fluorescence image showing the position of an astrocyte cell body and the locations in which a UV pulse was delivered to photo-release glutamate. (B1) No calcium response is detected in this cell body when the UV flash is delivered adjacent to the soma. (B2) A calcium response is detected when glutamate is photolytically delivered directly onto the cell body. All images are shown as ΔF values so that changes in fluorescence are observed on a dark background. (C) Summary of the changes in astrocytic fluo-4 fluorescence in response to photolysis adjacent to (position 1) and directly on (position 2) the astrocyte cell body (n=7). Color scale represents the linear pseudocolor scale (0–255). Scale bar: 20 μm, *** P<0.001.
To determine the speed at which calcium responses are transmitted between connected astrocytes we plotted stimulus–response delay histograms. From these, it is clear that the majority of astrocytes respond within 10–30 seconds of the stimulus (Fig. 6A). Therefore, we used a 30-second cut-off criterion to select calcium responses in connected cells that were caused by stimulation of the primary cell rather than spontaneous oscillations (Nett et al., 2002). Using this criterion to select responsive cells, we found that many of the connected astrocytes cluster around a modal value of 47 μm from the stimulated cell (Fig. 6B), with additional modes located at 74 μm and 107 μm. These modal values correspond to the distance that separates somata on astrocytes (Ogata and Kosaka, 2002). The average linear conduction velocity of delayed calcium responses was 10.7 ± 0.82 μm sec−1 (n=75).
Fig. 6. Delayed calcium elevations in functionally connected astrocytes are coupled to stimulation of the primary astrocyte.
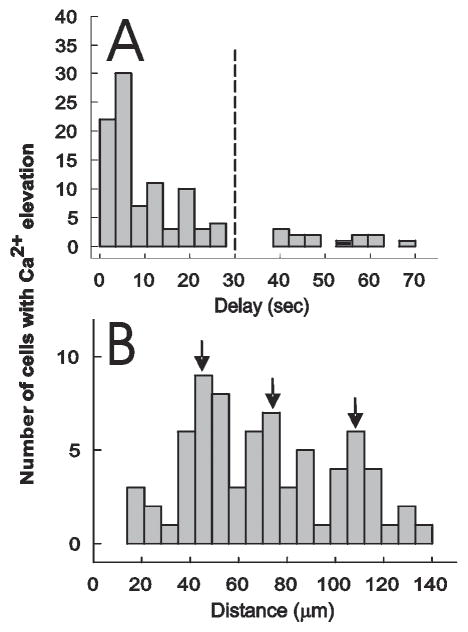
(A) Glutamate was photo-released onto an astrocyte and the delay before detecting a calcium response in neighboring cells in the imaging field determined. Because the latency to calcium response in nonstimulated cells is not randomly distributed, the stimulus-delay histogram shows that nonstimulated astrocytes are responding to activation of the primary astrocyte. The dashed line represents a 30-second-latency cut-off which we used to identify cells that are connected to the stimulated cell. (B) The distance from the stimulated astrocyte to functionally connected astrocytes (<30 second response latency). Note three modes of distance distribution that correspond to the radius of circumferentially located astrocytes.
To further characterize the properties of interconnected astrocytes we asked whether functional connections are unidirectional. After stimulating one astrocyte and determining which of its neighbors exhibited a calcium response, we reversed the direction of the stimulus and photolytically released glutamate onto neighboring cells to determine whether this induced a delayed calcium response in the cell that we stimulated originally. Despite the fact that astrocytes are interconnected by gap junctions, we found unidirectional connections between an average of 75.3 ± 10.4% of the cell pairs tested (12 pairs of cells in five hippocampal slices).
To see whether astrocytes interconnect via neuronal intermediates we blocked the ability to generate action potentials using TTX (10 μM; n=4) and prevented the release of synaptic transmitter by incubation in bafilomycin A1 (2.5 μM; n=9). Neither independent manipulation prevented calcium responses in connected astrocytes (data not shown). This supports the notion that these responses are mediated by direct connections between astrocytes rather than through neuronal intermediates.
To investigate whether calcium-dependent release of glutamate from astrocytes (Parpura and Haydon, 2000) mediates intercellular signaling between astrocytes, we stimulated one astrocyte directly using caged bradykinin (Givens et al., 2000) to see whether glutamate receptor antagonists blocked the delayed calcium responses in neighboring, connected astrocytes. We first applied bradykinin to brain slices to identify which astrocytes responded to this peptide. To prevent receptor desensitization in these studies we applied a low concentration of bradykinin (1 nM). We found that few astrocytes express functional bradykinin receptors (Fig. 7B), which agrees with previous immunocytochemistry (Chen et al., 2000). On average 7.5 ± 2.4% (n=4) of astrocytes responded to bradykinin with an immediate calcium response. Additionally, we detected delayed calcium responses in neighboring astrocytes (delay 6–20 seconds), which confirms our results with caged glutamate (Figs 4,5) that the cell that was stimulated directly evoked calcium signals in connected neighbors. Therefore, we targeted our photolysis point to cells that responded immediately to bradykinin and found that photo-release of bradykinin from caged bradykinin (10 nM) evoked a calcium signal in the bradykinin-responsive cell and a delayed response in 6.0 ± 1.4 (n=4) adjacent astrocytes (Fig. 7C). Because bath application of bradykinin showed that only the photo-stimulated cell responds directly to bradykinin, this result supports the notion that astrocytes are functionally coupled, and that delayed responses in neighbors are not merely caused by diffusion of photo-released transmitter. Incubating slices in the glutamate receptor antagonists MCPG (20 μM), CNQX (20 μM) and D-AP5 (50 μM) does not attenuate the ability of photo-released bradykinin to stimulate calcium signaling in connected astrocytes (Fig. 7C,D), which demonstrates that glutamate released from astrocytes in response to calcium elevations in astrocytes does not mediate this form of inter cellular signaling.
Fig. 7. Glutamate release from astrocytes does not mediate astrocyte connectivity.
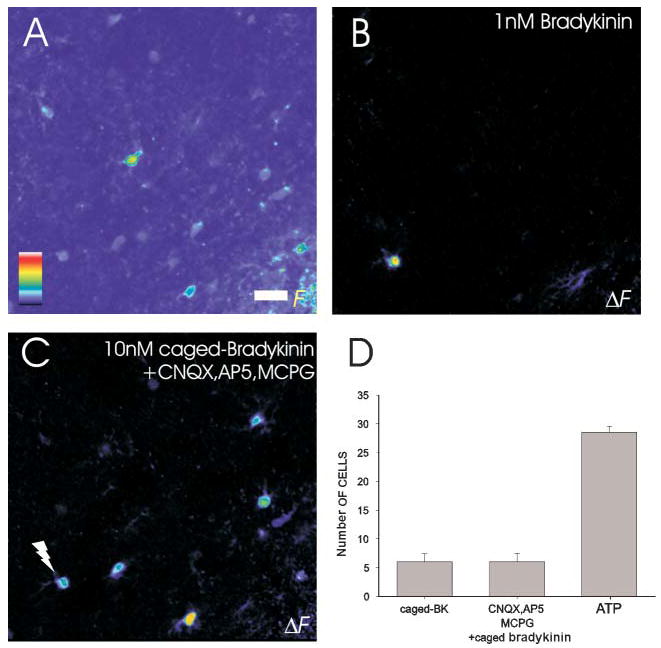
(A) A fluorescence image of astrocytes in the stratum radiatum to which 1 nM bradykinin was applied to identify bradykinin-responsive cells. (B) In this field of view, one astrocyte responds to bradykinin. (C) Photo-release of bradykinin onto this astrocyte in either the presence or absence of glutamate receptor antagonists CNQX, D-AP5 and MCPG (20 μM, 50 μM and 20 μM, respectively; n=4). (D) The bradykinin induced increase in calcium in this cell caused delayed calcium responses in four adjacent astrocytes that were unaffected by the glutamate receptor antagonists. Color scale represents the linear pseudocolor scale (0–255). Scale bar: 20 μm.
Cell-culture studies, as well as studies performed in the retina (Newman, 2001), show that inter-astrocytic calcium waves are mediated, in part at least, by the release of ATP (Cotrina et al., 1998; Guthrie et al., 1999; Wang et al., 2000). It is difficult to use antagonists to probe purinergic signaling in brain slices. The purinergic antagonists pyroxidal-5′-phosphate-6-azophenyl-2′4-disulphonic acid (PPADS) and suramin affect the direct calcium response to glutamate in the stimulated cell, which indicates a high-degree of receptor cross-talk (J-Y. Sul and P.G. Haydon, unpublished observations). Instead, we asked whether FFA, which reduces the release of ATP from astrocytes (Stout et al., 2002) reduces connectivity. FFA (200 μM; n=4) reduced significantly the number of connected astrocytes in brain slices, from a control value of 4.5 ± 1.0 to 1.0 ± 0.4, but did not change the ability of photo-released glutamate to initiate a calcium response in the directly stimulated astrocyte. Although FFA might have other actions, in addition to blocking ATP release, this observation is consistent with cell-culture studies that indicate that ATP released through FFA-sensitive channels mediates calcium signaling between connected cells (Stout et al., 2002).
A characteristic feature of synaptically connected neuronal circuits is their ability to be modulated. We asked whether the astrocytic circuits that we observed are fixed in nature, or whether other cells can be recruited into the circuit by subthreshold neurotransmitter concentrations. Although application of 1 μM ATP alone to a hippocampal slice caused no change in astrocytic calcium (Fig. 8D), when combined with photo-release of glutamate it recruited additional astrocytes into the circuits (Fig. 8A–C). Photo-release of glutamate onto one astrocyte led to calcium responses in 3.9 ± 0.8 (n=14) connected cells. However, when repeated in the presence of 1 μM ATP, 6.8 ± 0.9 cells were connected (Fig. 8B,C). Stimulated astrocytes were functionally connected to other astrocytes over a greater range in the presence of 1 μM ATP than in control conditions (Fig. 8E,F). Figure 8E shows the distance distribution of astrocytes that respond to each of two glutamate stimuli. Repeated responses are detected in cells distributed around a modal value of 47 μm from the stimulated cell. However, when ATP (1 μM) is present during the second glutamate stimulus, cells at a greater distance respond, with modal distances of 47 μm, 74 μm and 107 μm (Fig. 8F). Similarly, addition of ACh increased the number of connected astrocytes (data not shown).
Fig. 8.
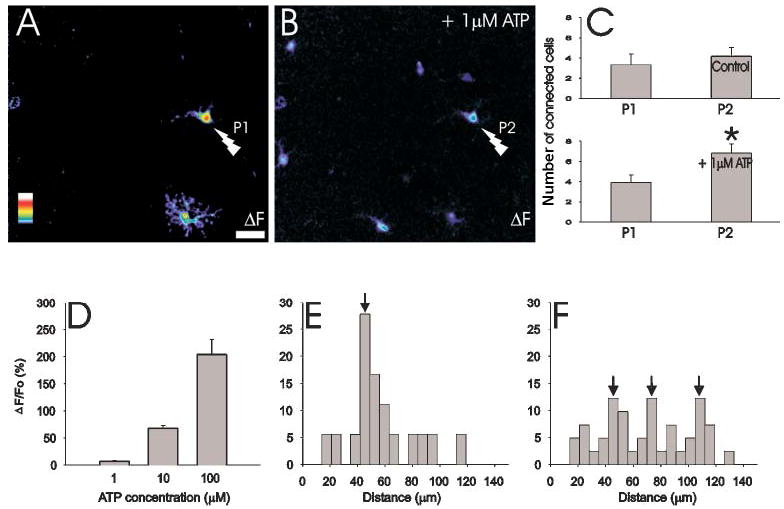
ATP augments the number of functionally connected astrocytes. (A) Maximal projection ”F image showing the peak change in fluorescence through a time-series of images. Photolysis (P1) evokes a calcium elevation in the directly stimulated cell (lightning bolt) and in one neighbor. (B) By itself, ATP (1 μM) does not change astrocytic calcium concentrations, but it increases the number of functionally connected astrocytes that respond to a second photolytic stimulus (P2) applied to the same primary astrocyte. (C) Whereas repeated stimulation of an astrocyte does not enhance connectivity, the presence of 1 μM ATP augments the number of connected astrocytes (n=14). (D) Dose-response relationship between the concentration of ATP and the change in fluo-4 fluorescence. Note that 1 μM ATP does not change the calcium concentration in astrocytes. (E) The distance distribution of functionally connected astrocytes that respond to both P1 and P2. (F) The distance distribution of astrocytes that respond to both P1 and P2 in the absence (P1) and presence of ATP (P2). Note that ATP enhances the reliability of functional connections to distant astrocytes. Color scale represents the linear pseudocolor scale (0–255). Scale bar: 20 μm, * P<0.02.
CONCLUSIONS
Subsets of adjacent astrocytes in hippocampal brain slice preparations interconnect functionally.
Subthreshold concentrations of the extracellular transmitters ATP and ACh increase the number of astrocytes that interconnect functionally.
DISCUSSION
This study demonstrates that physiological signals can activate astrocytic calcium signaling in brain slices and that, once activated, astrocytes communicate with one another through short-range connectivity. It is evident that the extent of these circuits is regulated dynamically by the extracellular milieu, because addition of subthreshold concentrations of either ATP or ACh modulates the number of interconnected cells.
The mechanism that underlies intercellular calcium signaling between astrocytes in brain slices is not clear but it is likely that ATP serves a role as an intercellular signal. Because an increase in intracellular calcium induces the release of glutamate from astrocytes (Parpura and Haydon, 2000; Bezzi et al., 1998), we first determined whether glutamate receptors are required for astrocytic connectivity. Because focal photo-release of bradykinin evokes calcium signals in neighboring, interconnected astrocytes, even in the presence of glutamate receptor antagonists, we conclude that the glutamate-release pathway does not mediate astrocytic connectivity. It is perhaps surprising that glutamate released from astrocytes does not mediate astrocyte connectivity. Although the reasons for this observation are unclear, it might be related to the very limited spatial range of the transmitter that results from the activity of glutamate transporters on the plasma membrane.
Previous cell culture studies show that both ATP and gap junctions have important roles in mediating calcium waves (Giaume and Venance, 1998; Guthrie et al., 1999; Stout et al., 2002; Cotrina et al., 1998). Long-range calcium signals require the release of ATP from astrocytes, whereas shorter-range calcium waves can be supported by ATP-independent mechanisms. In the former instance, it is thought that activation of a metabotropic receptor, such as mGluR, leads to PLC- and IP3-dependent release of calcium from internal stores of the stimulated cells. Activation of PLC also releases ATP from the stimulated cell (Wang et al., 2000), although details of the mechanism are debated. Released ATP diffuses to neighboring astrocytes where it activates P2Y receptors (Fam et al., 2000), which, in turn, activate PLC. This leads to the release of calcium from internal stores of the ATP-stimulated cell, and to the further release of ATP, which activates additional neighbors (Haydon, 2001). The concept that ATP release is crucial for the calcium wave is supported by evidence that ATP is released from astrocytes, that ATP induces astrocytic calcium signals, and that blockade of purinergic receptors reduces the extent of the calcium wave. Even when purinergic receptors are blocked, short-range calcium waves are detected, but the rate of propagation is reduced. It is believed that these waves are mediated by the diffusion of IP3 through gap junctions that connect astrocytes. However, in this study we found that the majority of cell pairs are connected unidirectionally, despite the presence of gap junctions. This makes it less likely that the signal that initiates the calcium wave spreads between neighbors through these intercellular junctions.
To determine whether purinergic systems support calcium waves we added purinergic receptor antagonists (e.g. PPADS and suramin) to brain-slice preparations. Such antagonists did block calcium waves, but they also reduced the ability of glutamate to induce calcium signals in the directly stimulated cell, which makes it impossible to interpret these experiments. Although not conclusive, support for a role of ATP in mediating calcium waves is provided by the observation that exogenous ATP at a concentration that, by itself, does not induce calcium signals augments the spread of the calcium signal, as indicated by the greater distance over which calcium signals are detected (Fig. 8F) and the increase in the number of cells that respond (Fig. 8C). Although our study indicates a role for ATP in mediating astrocyte–astrocyte signaling, we cannot discount a contribution from IP3 that diffuses through the gap junctions that interconnect astrocytes.
The ability to undergo modulation is a key feature of signaling systems in the nervous system, at the level of both individual cells and their signal transduction cascades and of intercellular signaling pathways and synaptic transmission. Addition of exogenous transmitters, ATP and Ach, augmented the number of astrocytes that responded to a calcium signal in a stimulated astrocyte. At least two mechanisms might mediate this modulation. First, exogenously applied transmitter might induce a change in the phosphorylation state of other receptors that are involved in mediating the intercellular spread of the calcium signal, which would lead to enhanced sensitivity to activation. Adenosine, for example, modulates the sensitivity of mGluR5 to initiate calcium signaling (Cormier et al., 2001). Second, low levels of exogenous transmitter might elevate the intracellular concentration of IP3 so that previously sub-threshold inputs to the cell become capable of initiating an IP3-dependent calcium signal. At present, it is not possible to discriminate between these possibilities, largely because technical limitations allow us to resolve only the calcium signal. This is much like trying to make inferences about changes in synaptic potentials from an extracellular recording of action potentials. Until we can record directly the spatio–temporal changes in IP3 levels that underlie the calcium signal, many of these questions will remain unanswered.
Regardless of the regulation mechanisms, this study does demonstrate that there is a dynamic continuum of calcium signaling within astrocytes. Previous studies have shown that calcium oscillations can be restricted either to microdomains or to individual processes of a single astrocyte (Grosche et al., 1999; Nett et al., 2002). These calcium signals can also spread throughout a whole astrocyte, as well as signal to immediate neighbors.
The extent of spread of this signal through interconnected astrocytes is regulated by transmitters in the extracellular environment. The functional consequences of regulating the spread of the calcium wave are likely to be numerous. Cell-culture studies show that hippocampal astrocytes release neurotransmitters in response to elevated intracellular calcium concentrations. Thus, the extent of spread of the calcium signal will regulate the overall extrasynaptic neuronal space affected by these glial-derived chemical transmitters.
The challenge is to determine the role of the short-range astrocytic calcium-signaling circuits in CNS function. There are several possibilities. For example, neuron-evoked astrocytic calcium signaling associated with the production of prostaglandin E2 might have important roles in controlling local blood flow (Zonta et al., 2003). Alternatively, astrocytic calcium signaling evokes the release of glutamate and ATP. By acting on extrasynaptic receptors these chemical transmitters cause synaptic modulation, thus, it is feasible that astrocytic circuits could lead to a spatial synchronization of synaptic transmission. Regardless of the specific function(s), calcium signaling within astrocytic circuits adds another layer of complexity to the control of the release of chemical transmitters in the nervous system.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institutes of Health (RO1 NS43142 and R37 NS37585). We wish to thank Daniel Evanko and Yolande Haydon for constructive comments on a version of the manuscript and Dr. Robert Zorec for his discussions of data analysis.
Footnotes
A movie showing the relative calcium concentrations in astrocytes from the stratum radiatum of the hippocampus following photo-release of caged glutamate onto a single astrocyte accompanier the online version of this article at http://journals.cambridge.org/jid-NGB.
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. European Journal of Neuroscience. 1998a;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. The Journal of Neuroscience. 1998b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999;22 :208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Chen EY, Emerich DF, Bartus RT, Kordower JH. B2 bradykinin receptor immunoreactivity in rat brain. The Journal of Comparative Neurology. 2000;427:1–18. [PubMed] [Google Scholar]
- Cormier RJ, Mennerick S, Melbostad H, Zorumski CF. Basal levels of adenosine modulate mGluR5 on rat hippocampal astrocytes. Glia. 2001;33:24–35. doi: 10.1002/1098-1136(20010101)33:1<24::aid-glia1003>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proceedings of the National Academy of Sciences,USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y(1) purinoceptor-mediated Ca(2+) signaling and Ca(2+) wave propagation in dorsal spinal cord astrocytes. The Journal of Neuroscience. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- Givens RS, Weber JF, Conrad PG, Orosz G, Donahue SL, Thayer SA. New phototriggers 9: p-hydroxyphenacyl as a C-terminal photoremovable protcting group for oligopeptides. Journal of the American Chemical Society. 2000;122:2687–2697. [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuronglia interaction: parallel fiber signaling to Bergmann glial cells. Nature Neuroscience. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. The Journal of Neuroscience. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Zanotti SA, Frautschy SA, Charles AC. Spiral intercellular calcium waves in hippocampal slice cultures. Journal of Neurophysiology. 1998;79:1045–1052. doi: 10.1152/jn.1998.79.2.1045. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature Neuroscience. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. Journal of Biochemistry (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. Journal of Neurophysiology. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. The Journal of Neuroscience. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. The Journal of Neuroscience. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proceedings of the National Academy of Sciences, U.S.A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. The Journal of Neuroscience. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. The Journal of Neuroscience. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. The FASEB Journal. 2002;16:255–257. doi: 10.1096/fj.01-0514fje. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. The Journal of Biological Chemistry. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends in Neurosciences. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Volterra A., Magistretti P.J. and Haydon P.G. (2002) The Tripartite Synapse: Glia in Synaptic Transmission. Oxford University Press.
- Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Analytical Chemistry. 2000;72:2001–2007. doi: 10.1021/ac9912146. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Developmental Biology. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


