Abstract
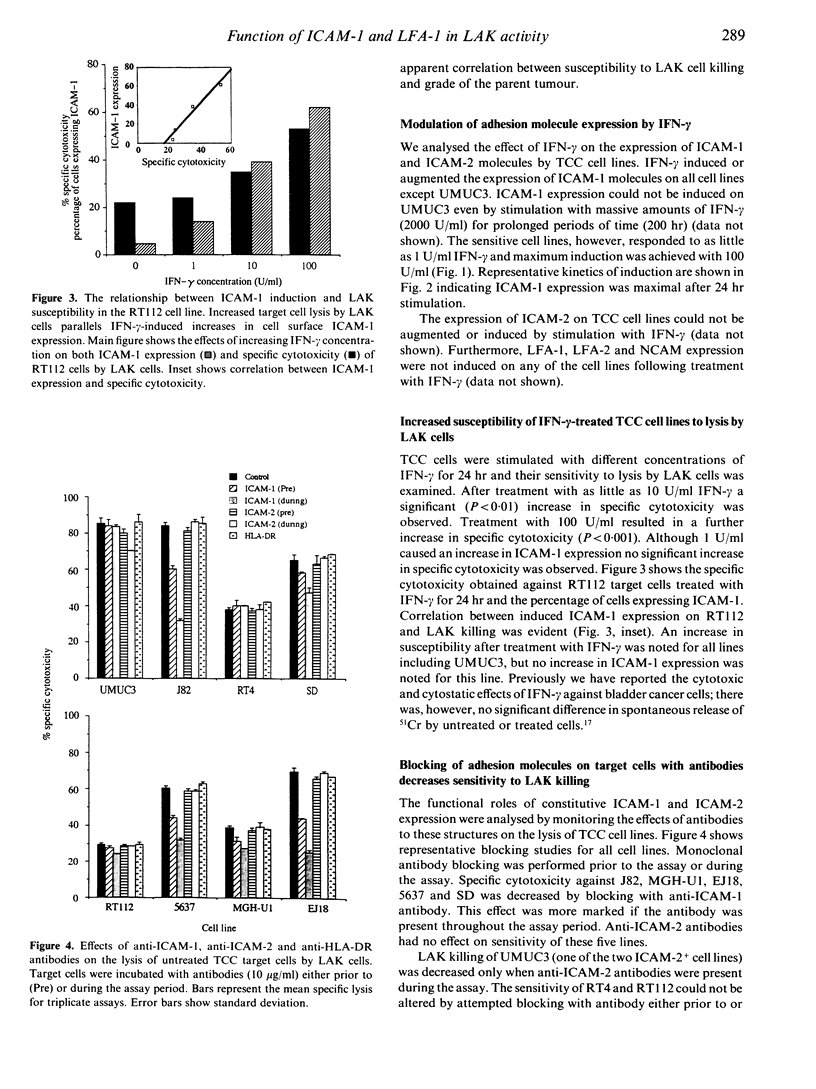
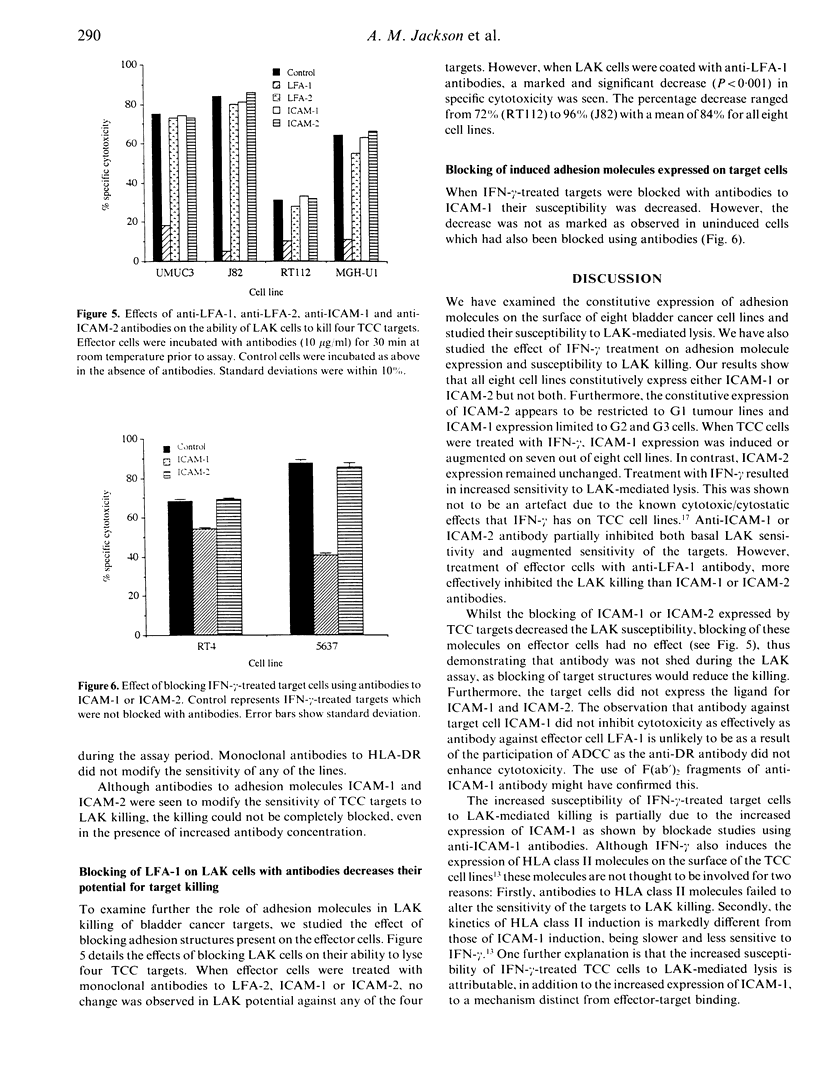
The lysis of eight human bladder cancer cell lines by lymphokine-activated killer cells (LAK) was found to be partially dependent upon the expression by the target cell of either intercellular adhesion molecule-1 (ICAM-1) or intercellular adhesion molecule-2 (ICAM-2). Using adhesion blockade studies these molecules were found to contribute towards sensitivity to lysis. Tumour lines of low grade (G1) did not constitutively express ICAM-1, but were found to express ICAM-2. High grade cells (G2, G3), however, only constitutively expressed ICAM-1 on their cell surface. Interferon-gamma (IFN-gamma) induced and augmented the expression of ICAM-1 by all except one of the cell lines (UMUC3) in a dose- and time-dependent manner. This was accompanied by an increased susceptibility to lymphokine-activated killer mediated cytolysis. Anti-ICAM-1 antibodies partially inhibited the increase in cell lysis due to IFN-gamma. However, this inhibition was not complete. When effector cells were treated with antibodies to leucocyte function-associated antigen-1 (LFA-1) the inhibition of lysis was greater and ranged from 72 to 96% with a mean of 87% inhibition. These results suggest that the increased sensitivity of IFN-gamma-treated bladder cancer cell lines to LAK cells is partially attributable to the induction of ICAM-1. However, blocking of ICAM-1 with antibodies could only partially inhibit the increased LFA-1-dependent lysis. This supports recent evidence for the existence of an additional ligand for LFA-1, other than ICAM-1 and ICAM-2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönberg A., Ferm M. T., Ng J., Reynolds C. W., Ortaldo J. R. IFN-gamma treatment of K562 cells inhibits natural killer cell triggering and decreases the susceptibility to lysis by cytoplasmic granules from large granular lymphocytes. J Immunol. 1988 Jun 15;140(12):4397–4402. [PubMed] [Google Scholar]
- Grönberg A., Ferm M., Tsai L., Kiessling R. Interferon is able to reduce tumor cell susceptibility to human lymphokine-activated killer (LAK) cells. Cell Immunol. 1989 Jan;118(1):10–21. doi: 10.1016/0008-8749(89)90353-5. [DOI] [PubMed] [Google Scholar]
- Hawkyard S., James K., Prescott S., Jackson A. M., Ritchie A. W., Smyth J. F., Chisholm G. D. The effects of recombinant human interferon-gamma on a panel of human bladder cancer cell lines. J Urol. 1991 May;145(5):1078–1081. doi: 10.1016/s0022-5347(17)38538-5. [DOI] [PubMed] [Google Scholar]
- Jackson A. M., Hawkyard S. J., Prescott S., Ritchie A. W., James K., Chisholm G. D. An investigation of factors influencing the in vitro induction of LAK activity against a variety of human bladder cancer cell lines. J Urol. 1992 Jan;147(1):207–211. doi: 10.1016/s0022-5347(17)37198-7. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A. F., Lightfoot E., Lipsky P. E., Oppenheimer-Marks N. Role of CD11/CD18 in adhesion and transendothelial migration of T cells. Analysis utilizing CD18-deficient T cell clones. J Immunol. 1991 Jun 15;146(12):4149–4156. [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities. Scand J Immunol. 1989 Aug;30(2):129–164. doi: 10.1111/j.1365-3083.1989.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A., Cavarec L., Kermarrec N., Marchiol-Fournigault C., Gil M. L., Conjeaud H., Fradelizi D. Target lysis by human LAK cells is critically dependent upon target binding properties, but LFA-1, LFA-3 and ICAM-1 are not the major adhesion ligands on targets. Int J Cancer. 1991 Feb 1;47(3):473–479. doi: 10.1002/ijc.2910470328. [DOI] [PubMed] [Google Scholar]
- Rivoltini L., Cattoretti G., Arienti F., Mastroianni A., Melani C., Colombo M. P., Parmiani G. The high lysability by LAK cells of colon-carcinoma cells resistant to doxorubicin is associated with a high expression of ICAM-1, LFA-3, NCA and a less-differentiated phenotype. Int J Cancer. 1991 Mar 12;47(5):746–754. doi: 10.1002/ijc.2910470521. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- Symington F. W., Santos E. B. Lysis of human keratinocytes by allogeneic HLA class I-specific cytotoxic T cells. Keratinocyte ICAM-1 (CD54) and T cell LFA-1 (CD11a/CD18) mediate enhanced lysis of IFN-gamma-treated keratinocytes. J Immunol. 1991 Apr 1;146(7):2169–2175. [PubMed] [Google Scholar]
- Wang P., Vánky F., Li S. L., Patarroyo M., Klein E. Functional characteristics of the intercellular adhesion molecule-1 (CD54) expressed on cytotoxic human blood lymphocytes. Cell Immunol. 1990 Dec;131(2):366–380. doi: 10.1016/0008-8749(90)90261-o. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fries R. U., Golub S. H. Characteristics and mechanism of IFN-gamma-induced protection of human tumor cells from lysis by lymphokine-activated killer cells. J Immunol. 1988 May 15;140(10):3686–3693. [PubMed] [Google Scholar]


