Abstract
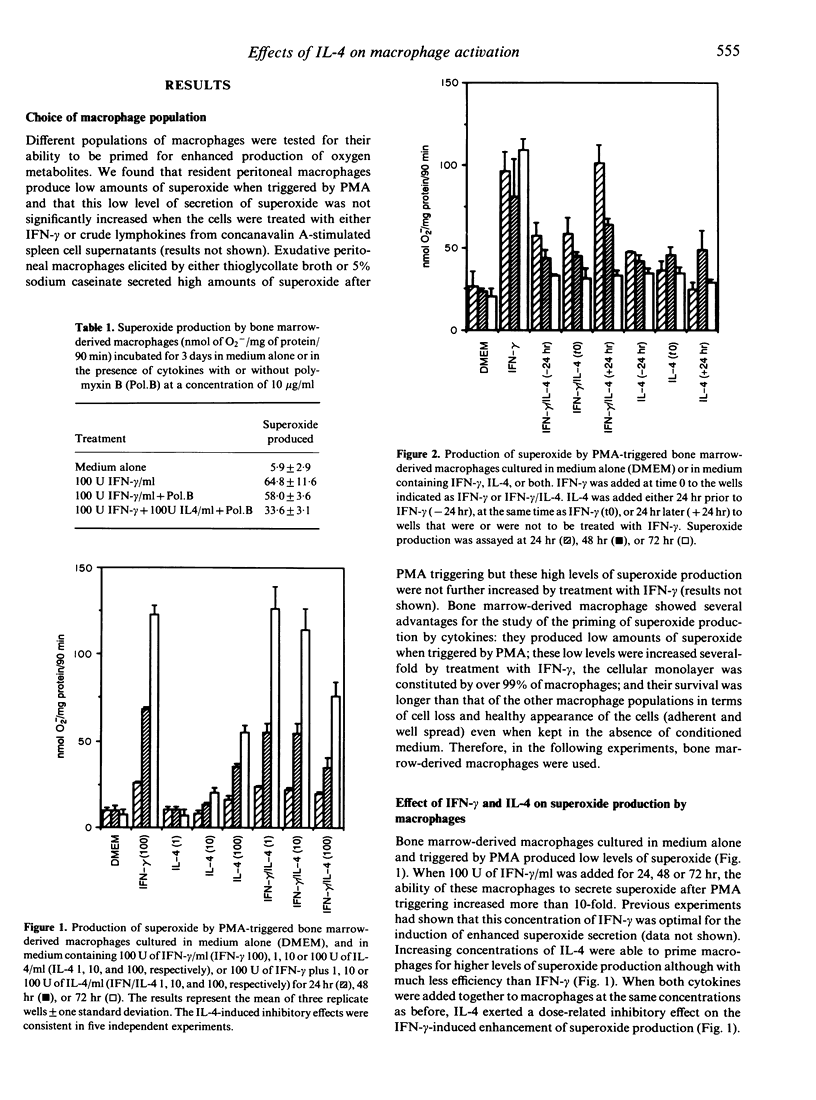
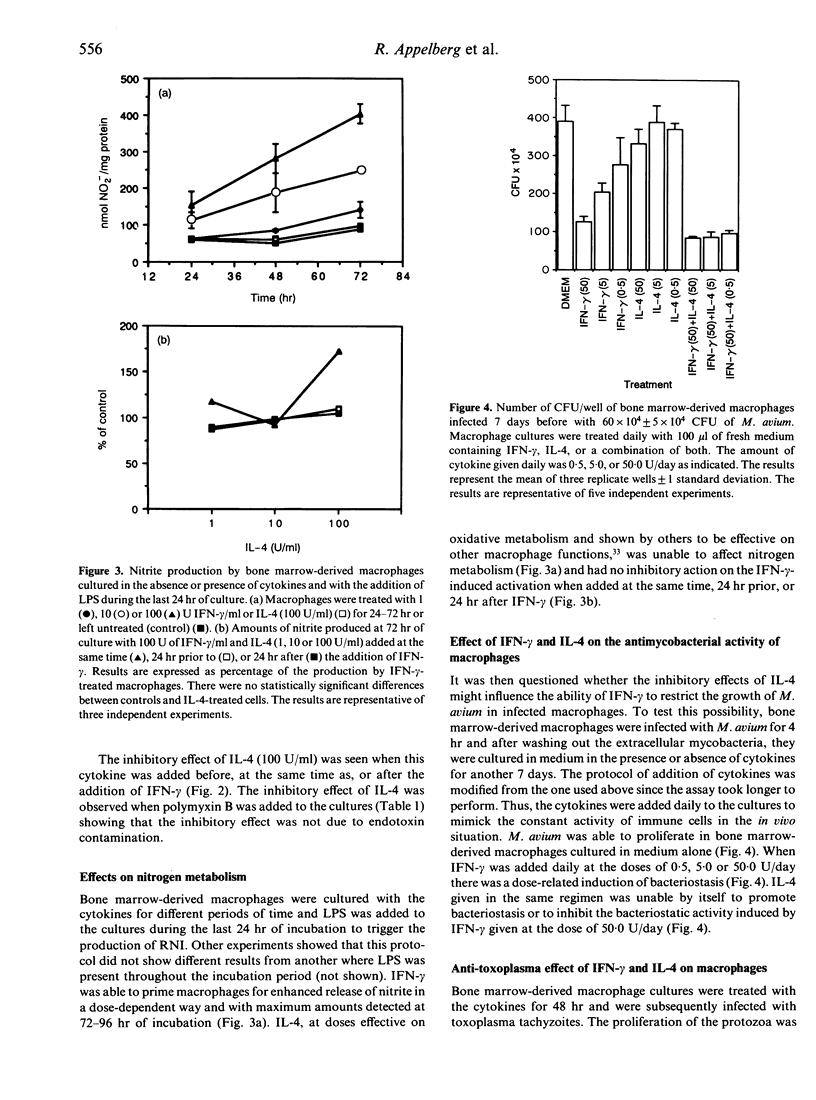
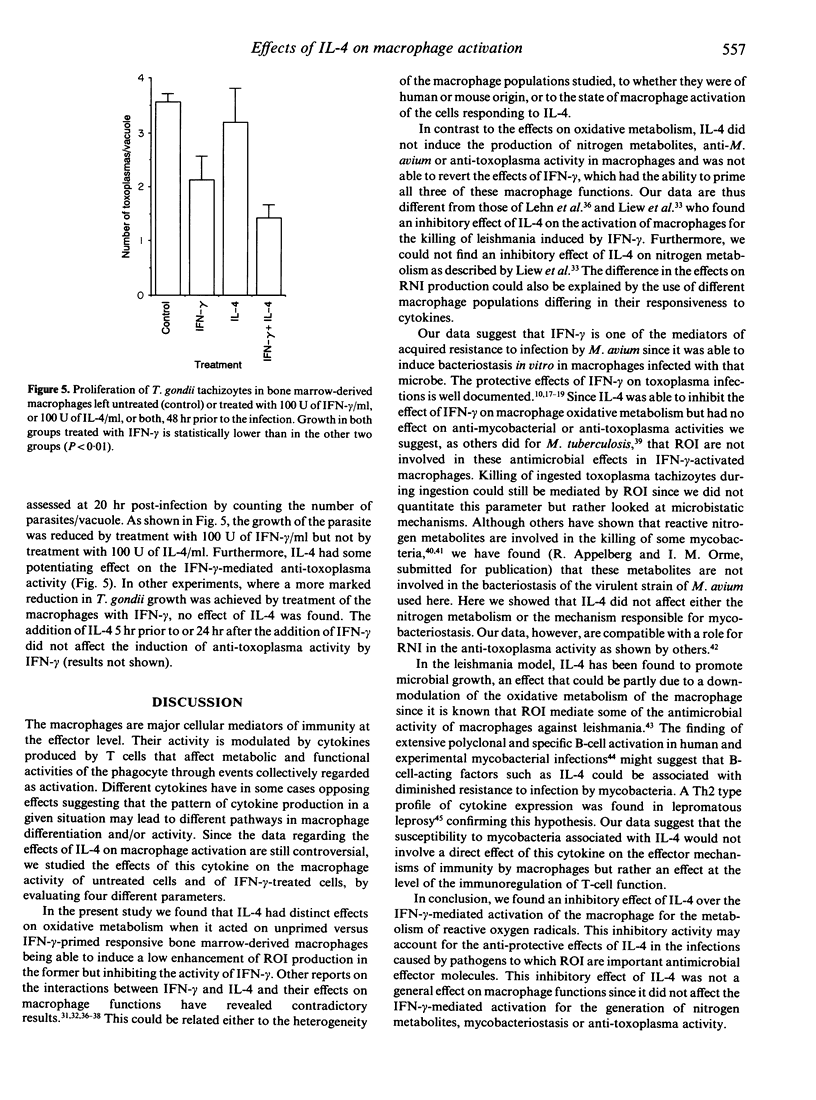
Interferon-gamma (IFN-gamma) and interleukin-4 (IL-4) have been shown to be secreted by distinct T-helper cell subsets which have different roles in the determination of host resistance to infection. We studied the activity of these two cytokines on effector mechanisms of mouse macrophages. In vitro cultured bone marrow-derived macrophages from C57BL/6 mice were treated with IFN-gamma, IL-4, or a combination of both cytokines and the ability to secrete superoxide or nitrite or to restrict growth of Mycobacterium avium and Toxoplasma gondii was then evaluated. We found that IL-4 could inhibit the priming of macrophages for enhanced superoxide production induced by IFN-gamma although IL-4 when used alone did have some enhancing effect of its own. This effect of IL-4 on IFN-gamma-primed superoxide production was dose dependent and could be observed even if the treatment by IL-4 was done 24 hr after treatment by IFN-gamma. IL-4 did not, however, influence the enhanced production of nitrogen reactive intermediates, the induction of bacteriostatic activity against M. avium, or the restriction of T. gondii by IFN-gamma-treated macrophages, and did not have any effect of its own regarding these latter functions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. L., Gallin J. I. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol. 1990 Jan 15;144(2):625–630. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger A. M., Hutchman J. S., Sung C. P., Bugelski P. J. Activation of rat alveolar macrophages by gamma interferon to inhibit Toxoplasma gondii in vitro. J Leukoc Biol. 1987 Nov;42(5):447–454. doi: 10.1002/jlb.42.5.447. [DOI] [PubMed] [Google Scholar]
- Becker S., Daniel E. G. Antagonistic and additive effects of IL-4 and interferon-gamma on human monocytes and macrophages: effects on Fc receptors, HLA-D antigens, and superoxide production. Cell Immunol. 1990 Sep;129(2):351–362. doi: 10.1016/0008-8749(90)90211-9. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988 Jun;56(6):1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Severn A., Millott S., Schmidt J., Salter M., Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991 Oct;21(10):2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. IV. Role of endogenous scavengers of oxygen intermediates. J Exp Med. 1980 Dec 1;152(6):1610–1624. doi: 10.1084/jem.152.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988 Jun 1;140(11):3978–3981. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Peleman R., Wu J., Fargeas C., Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989 Nov 1;170(5):1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Hamilton J. A. Priming the macrophage respiratory burst with IL-4: enhancement with TNF-alpha but inhibition by IFN-gamma. Immunology. 1990 Aug;70(4):498–503. [PMC free article] [PubMed] [Google Scholar]
- Ramos T., Zalcberg-Quintana I., Appelberg R., Sarno E. N., Silva M. T. T-helper cell subpopulations and the immune spectrum of leprosy. Int J Lepr Other Mycobact Dis. 1989 Mar;57(1):73–81. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. K., Banerjee D. K. Effect of gamma interferon on hydrogen peroxide production by cultured mouse peritoneal macrophages. Infect Immun. 1986 Nov;54(2):597–599. doi: 10.1128/iai.54.2.597-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Gallati H., Sinigaglia F., Walz A., Garotta G. Identity between human interferon-gamma and "macrophage-activating factor" produced by human T lymphocytes. Eur J Immunol. 1986 Dec;16(12):1471–1477. doi: 10.1002/eji.1830161202. [DOI] [PubMed] [Google Scholar]
- Wagner F., Fischer N., Lersch C., Hart R., Dancygier H. Interleukin 4 inhibits the interleukin 2-induced production of its functional antagonist, interferon gamma. Immunol Lett. 1989 Jun 1;21(3):237–241. doi: 10.1016/0165-2478(89)90110-7. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Sonnenfeld G., Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985 Jul;49(1):61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Zlotnik A. Effects of IL-4 on macrophage functions: increased uptake and killing of a protozoan parasite (Trypanosoma cruzi). Immunology. 1989 Feb;66(2):296–301. [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]


