Abstract
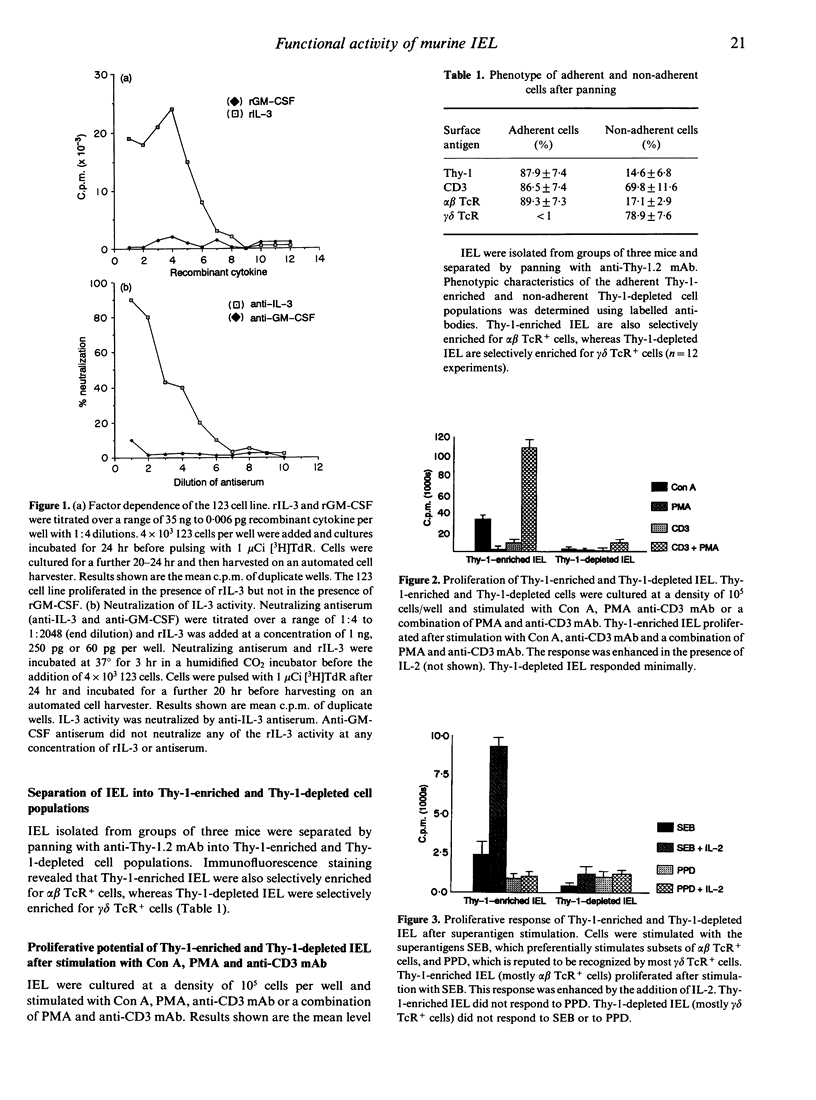
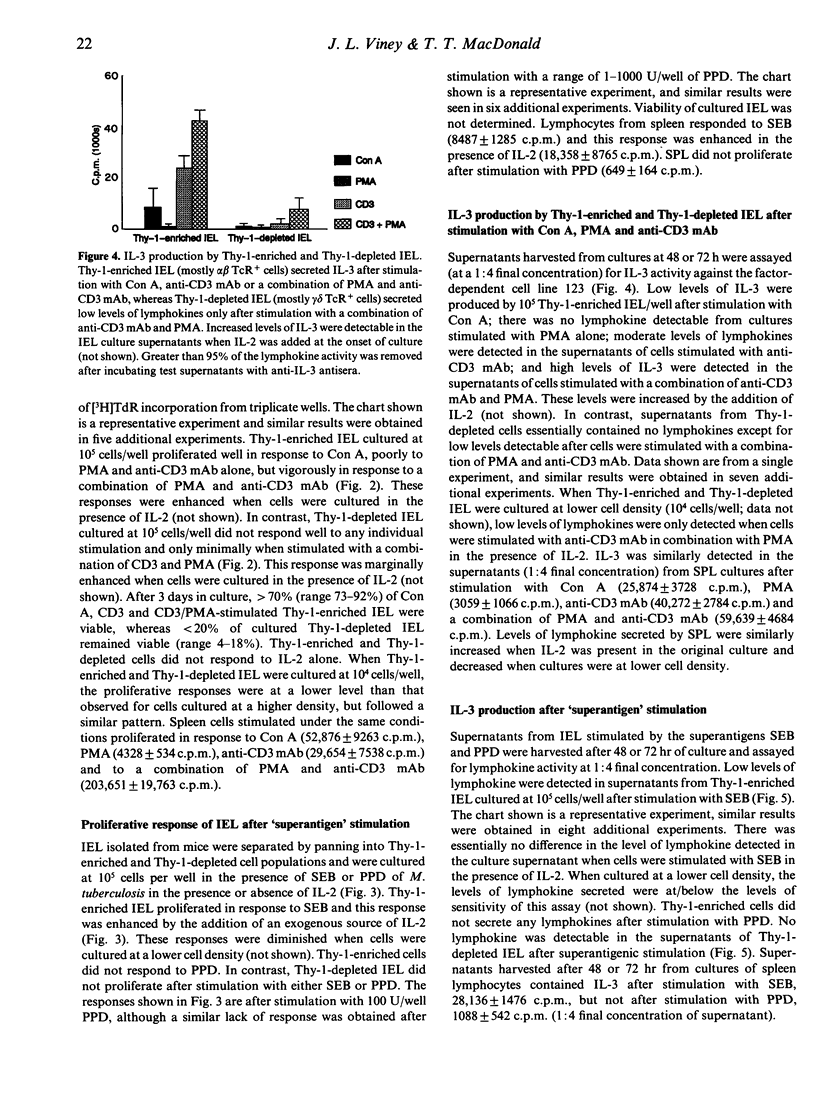
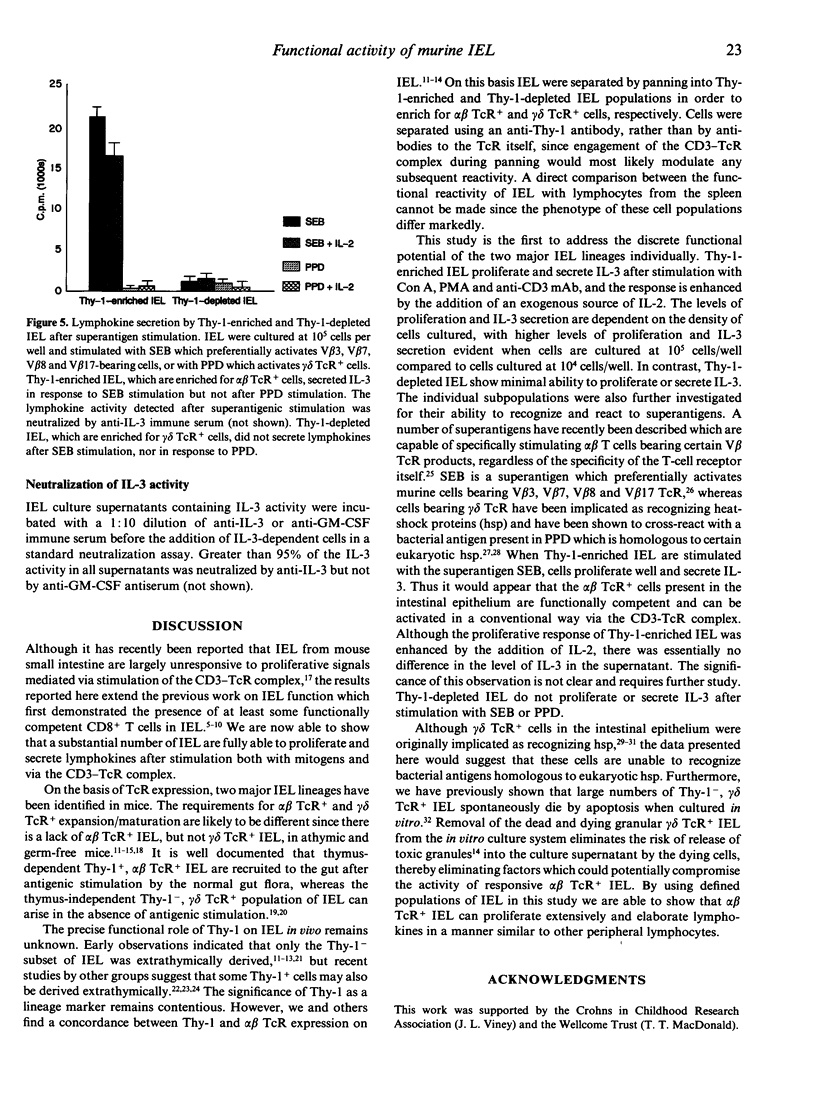
Compared to other peripheral lymphocytes, intestinal intraepithelial lymphocytes (IEL) have previously been shown to have low proliferative capabilities. However, there are two main populations of IEL in the small intestine of mice. First, there is the thymus-dependent CD3+,Thy-1+ population, most of which expresses the alpha beta T-cell receptor (TcR), and second there is the thymus-independent CD3+,Thy-1- population, most of which expresses the gamma delta TcR. In this study Thy-1-enriched and Thy-1-depleted lymphocytes from murine intestinal epithelium were studied separately for their ability to proliferate and secrete lymphokines in vitro after mitogenic stimulation, after stimulation via the TcR-CD3 complex and after stimulation with the superantigen Staphylococcus aureus B (SEB). Here we show that Thy-1-enriched IEL are not an immunocompromised population of cells but are functionally competent T cells that are capable of proliferation and lymphokine secretion after stimulation with concanavalin A (Con A), phorbol myristate acetate (PMA) and anti-CD3 monoclonal antibody (mAb). Furthermore, Thy-1-enriched IEL proliferate and secrete lymphokines after 'superantigenic' stimulation with SEB. In contrast, the majority of Thy-1-depleted IEL do not proliferate, and secrete only minimal levels of lymphokine to any of the stimuli tested in this study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Itohara S., Krecko E. G., Mombaerts P., Ishida I., Katsuki M., Berns A., Farr A. G., Janeway C. A., Jr, Tonegawa S. Transgenic mice demonstrate that epithelial homing of gamma/delta T cells is determined by cell lineages independent of T cell receptor specificity. J Exp Med. 1990 Apr 1;171(4):1015–1026. doi: 10.1084/jem.171.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- De Geus B., Van den Enden M., Coolen C., Nagelkerken L., Van der Heijden P., Rozing J. Phenotype of intraepithelial lymphocytes in euthymic and athymic mice: implications for differentiation of cells bearing a CD3-associated gamma delta T cell receptor. Eur J Immunol. 1990 Feb;20(2):291–298. doi: 10.1002/eji.1830200210. [DOI] [PubMed] [Google Scholar]
- Dillon S. B., Dalton B. J., MacDonald T. T. Lymphokine production by mitogen and antigen activated mouse intraepithelial lymphocytes. Cell Immunol. 1986 Dec;103(2):326–338. doi: 10.1016/0008-8749(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional characterization of Con A-responsive Lyt2-positive mouse small intestinal intraepithelial lymphocytes. Immunology. 1986 Nov;59(3):389–396. [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984 Jul;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986 Sep;8(5):503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Ebert E. C. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989 Dec;97(6):1372–1381. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Roberts A. I., Brolin R. E., Raska K. Examination of the low proliferative capacity of human jejunal intraepithelial lymphocytes. Clin Exp Immunol. 1986 Jul;65(1):148–157. [PMC free article] [PubMed] [Google Scholar]
- Ernst P. B., Petit A., Befus A. D., Clark D. A., Rosenthal K. L., Ishizaka T., Bienenstock J. Murine intestinal intraepithelial lymphocytes II. Comparison of freshly isolated and cultured intraepithelial lymphocytes. Eur J Immunol. 1985 Mar;15(3):216–221. doi: 10.1002/eji.1830150303. [DOI] [PubMed] [Google Scholar]
- Greenwood J. H., Austin L. L., Dobbins W. O., 3rd In vitro characterization of human intestinal intraepithelial lymphocytes. Gastroenterology. 1983 Nov;85(5):1023–1035. [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr Frontiers of the immune system. Nature. 1988 Jun 30;333(6176):804–806. doi: 10.1038/333804a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Maloy K. J., Mowat A. M., Zamoyska R., Crispe I. N. Phenotypic heterogeneity of intraepithelial T lymphocytes from mouse small intestine. Immunology. 1991 Apr;72(4):555–562. [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Whetsell M., Klein J. R. Proliferative properties of murine intestinal intraepithelial lymphocytes (IEL): IEL expressing TCR alpha beta or TCR tau delta are largely unresponsive to proliferative signals mediated via conventional stimulation of the CD3-TCR complex. Int Immunol. 1991 Jun;3(6):563–569. doi: 10.1093/intimm/3.6.563. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., MacKenzie S., Baca M. E., Felstein M. V., Parrott D. M. Functional characteristics of intraepithelial lymphocytes from mouse small intestine. II. In vivo and in vitro responses of intraepithelial lymphocytes to mitogenic and allogeneic stimuli. Immunology. 1986 Aug;58(4):627–634. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., McInnes I. B., Parrott D. M. Functional properties of intra-epithelial lymphocytes from mouse small intestine. IV. Investigation of the proliferative capacity of IEL using phorbol ester and calcium ionophore. Immunology. 1989 Mar;66(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991 Feb 1;173(2):483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., Kilshaw P. J., MacDonald T. T. Cytotoxic alpha/beta+ and gamma/delta+ T cells in murine intestinal epithelium. Eur J Immunol. 1990 Jul;20(7):1623–1626. doi: 10.1002/eji.1830200734. [DOI] [PubMed] [Google Scholar]
- Viney J. L., MacDonald T. T., Kilshaw P. J. T-cell receptor expression in intestinal intra-epithelial lymphocyte subpopulations of normal and athymic mice. Immunology. 1989 Apr;66(4):583–587. [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., MacDonald T. T. Selective death of T cell receptor gamma/delta+ intraepithelial lymphocytes by apoptosis. Eur J Immunol. 1990 Dec;20(12):2809–2812. doi: 10.1002/eji.1830201242. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Ishida A., Murosaki S., Ando T., Nomoto K. Sequential appearance of T-cell receptor gamma delta- and alpha beta-bearing intestinal intra-epithelial lymphocytes in mice after irradiation. Immunology. 1991 Dec;74(4):583–588. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]


