Abstract
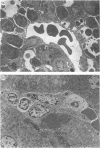
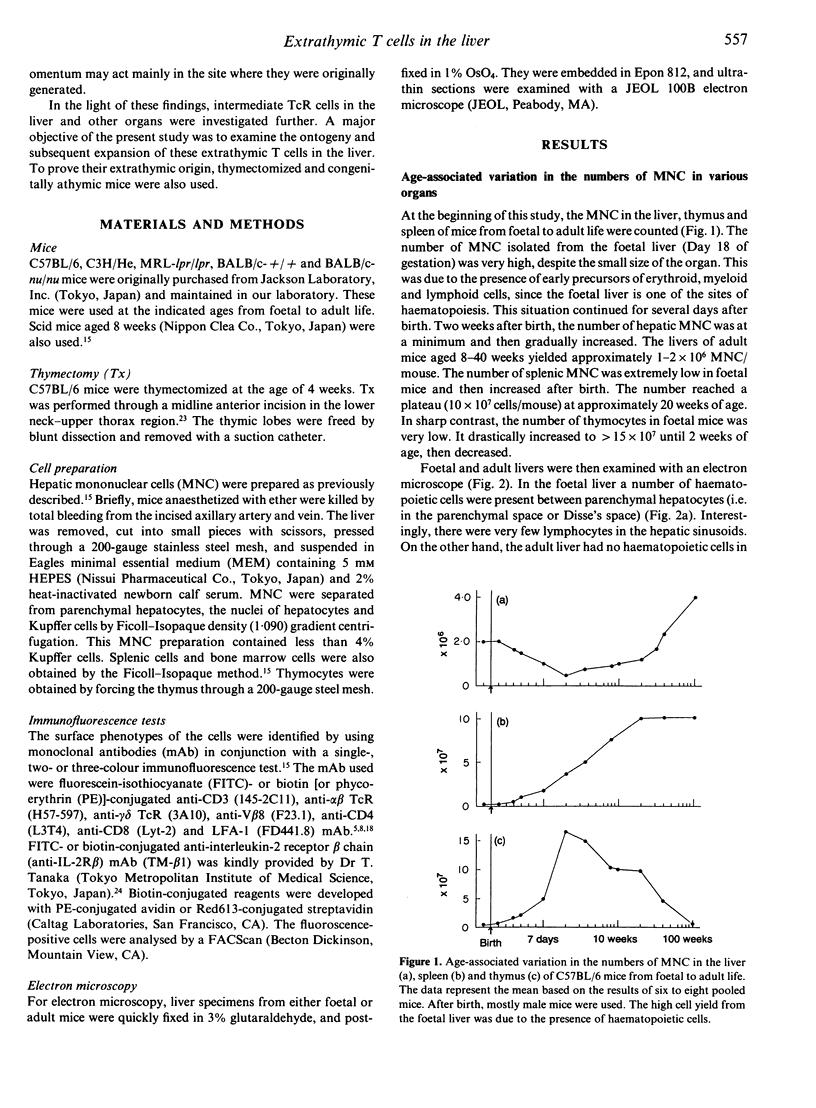
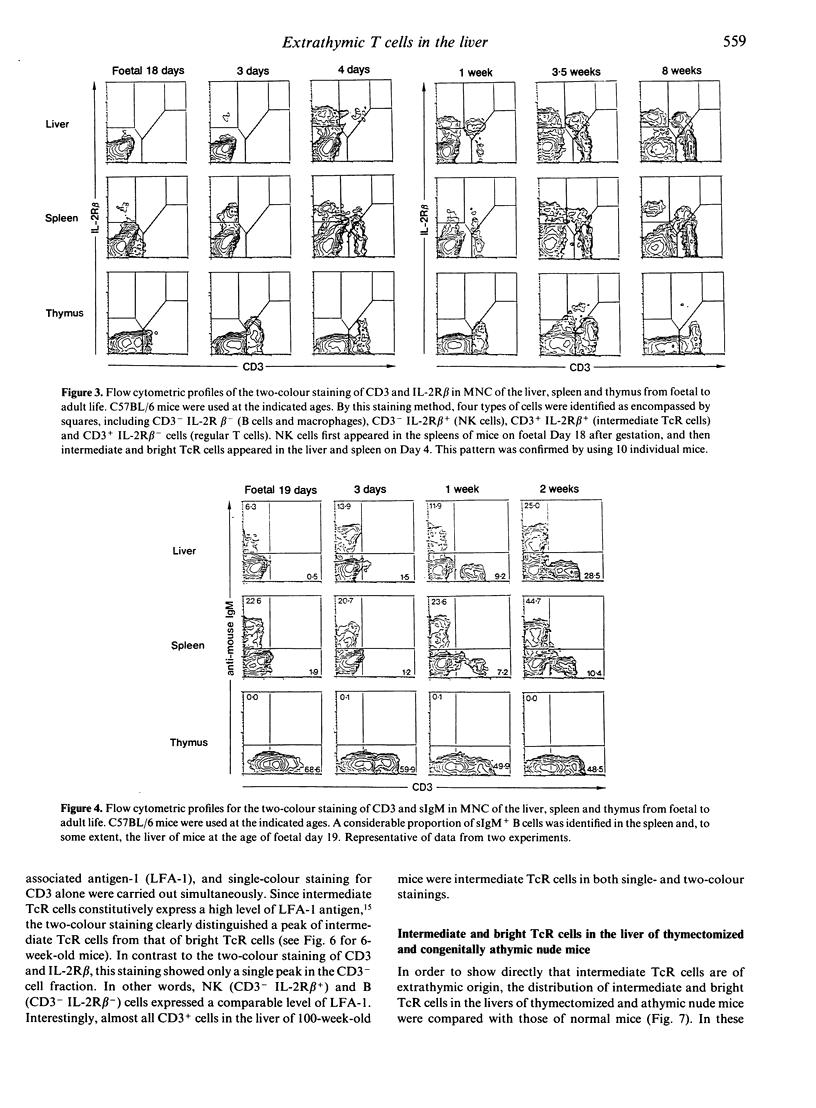
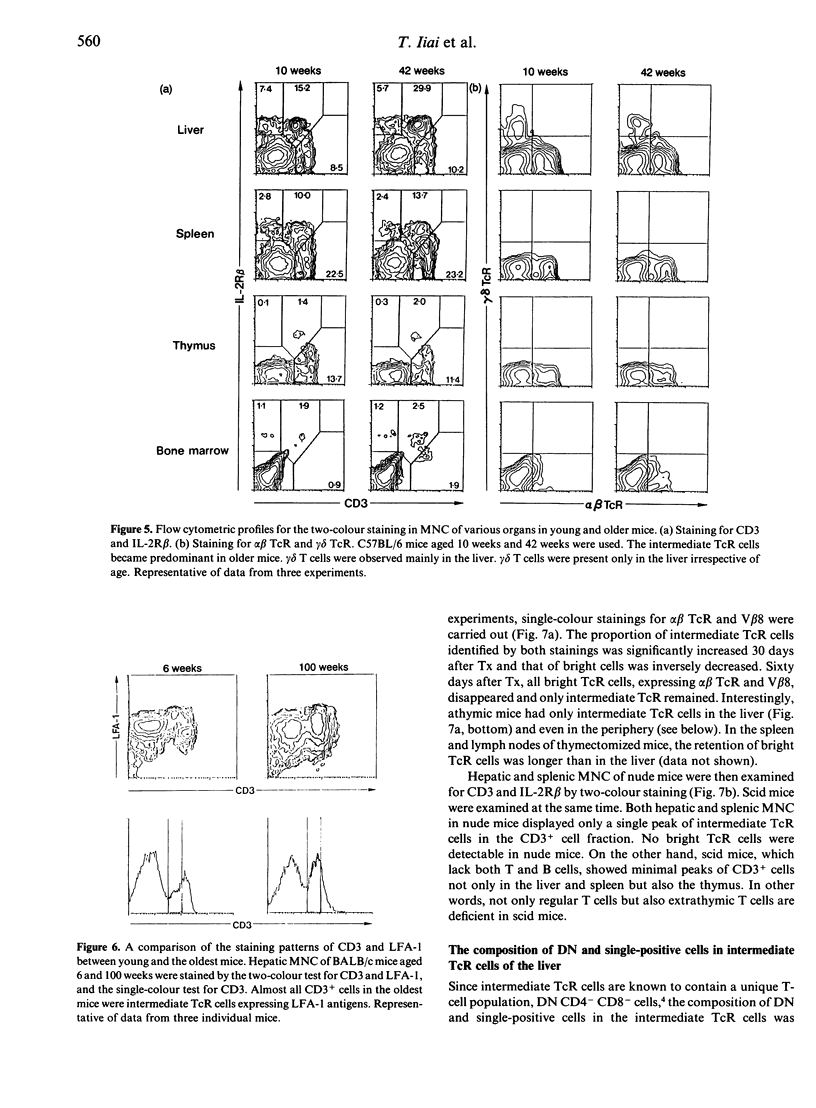
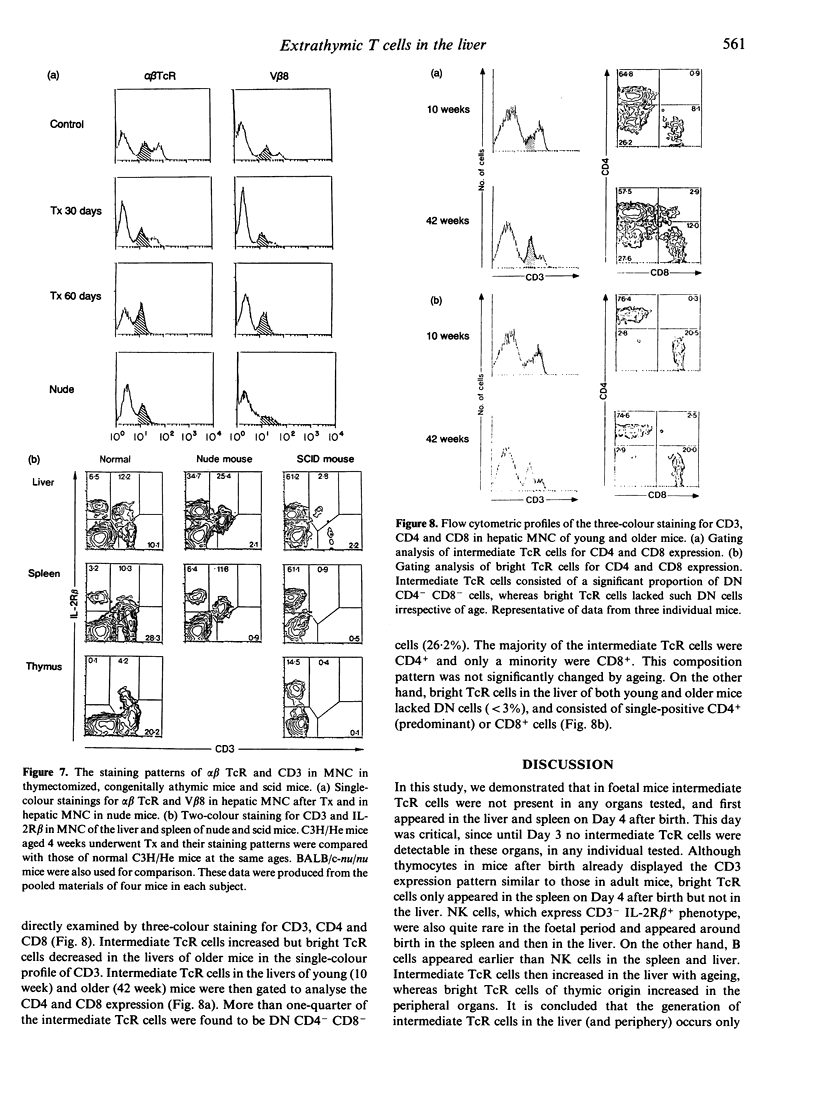
We previously demonstrated that the liver may be a major site of extrathymic T-cell differentiation in mice. In the present study, the ontogeny and subsequent development of such T cells in the liver and other organs were investigated. This study was possible because these T cells have T-cell receptors (TcR) of intermediate intensity (i.e. intermediate TcR cells) and constitutively express a high level of interleukin-2 receptor beta chain (IL-2R beta). Therefore the two-colour staining for CD3 (or alpha beta TcR) and IL-2R beta identifies even a small proportion of intermediate TcR cells. The total numbers of mononuclear cells obtained from the liver, thymus and spleen varied from foetal to adult life. Especially in the liver, many haematopoietic cells were present in the parenchymal space at the foetal stage. There were no lymphocytes in the sinusoidal lumen at this period. In contrast, lymphocytes appeared in the hepatic sinusoids after birth and increased with ageing. Phenotypic analysis revealed that intermediate TcR cells appeared in the liver and spleen on Day 4 after birth. Bright TcR cells of thymic origin were also present in the peripheral organs on Day 4. Thereafter, intermediate TcR cells increased in the liver, whereas bright TcR cells increased in the periphery as a function of age. Similarly, thymectomized and congenitally athymic mice had mainly intermediate TcR cells in the liver and, to some extent, periphery. It is concluded that intermediate TcR cells, possibly of extrathymic origin, are generated only after birth and expand with ageing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Sánchez J. L., Moreno de Alborán I. M., Marcos M. A., Sánchez-Movilla A., Martínez-A C., Kroemer G. Interleukin 2 abrogates the nonresponsive state of T cells expressing a forbidden T cell receptor repertoire and induces autoimmune disease in neonatally thymectomized mice. J Exp Med. 1991 Jun 1;173(6):1323–1329. doi: 10.1084/jem.173.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geus B., Van den Enden M., Coolen C., Nagelkerken L., Van der Heijden P., Rozing J. Phenotype of intraepithelial lymphocytes in euthymic and athymic mice: implications for differentiation of cells bearing a CD3-associated gamma delta T cell receptor. Eur J Immunol. 1990 Feb;20(2):291–298. doi: 10.1002/eji.1830200210. [DOI] [PubMed] [Google Scholar]
- Fangmann J., Schwinzer R., Wonigeit K. Unusual phenotype of intestinal intraepithelial lymphocytes in the rat: predominance of T cell receptor alpha/beta+/CD2- cells and high expression of the RT6 alloantigen. Eur J Immunol. 1991 Mar;21(3):753–760. doi: 10.1002/eji.1830210331. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Jones L. A., Kruisbeek A. M., Matis L. A. Thymic requirement for clonal deletion during T cell development. Science. 1989 Nov 24;246(4933):1044–1046. doi: 10.1126/science.2511630. [DOI] [PubMed] [Google Scholar]
- Itoh H., Abo T., Sugawara S., Kanno A., Kumagai K. Age-related variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunol. 1988 Jul 1;141(1):315–323. [PubMed] [Google Scholar]
- Kung J. T. Impaired clonal expansion in athymic nude CD8+CD4- T cells. J Immunol. 1988 Jun 1;140(11):3727–3735. [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Bron C., Sordat B., Miescher G. T cell antigen receptor expression in athymic (nu/nu) mice. Evidence for an oligoclonal beta chain repertoire. J Exp Med. 1987 Jul 1;166(1):195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Styre D., Klein J. R. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int Immunol. 1990;2(4):361–365. doi: 10.1093/intimm/2.4.361. [DOI] [PubMed] [Google Scholar]
- Murosaki S., Yoshikai Y., Ishida A., Nakamura T., Matsuzaki G., Takimoto H., Yuuki H., Nomoto K. Failure of T cell receptor V beta negative selection in murine intestinal intra-epithelial lymphocytes. Int Immunol. 1991 Oct;3(10):1005–1013. doi: 10.1093/intimm/3.10.1005. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Itoh T. Higher level expression of lymphocyte function-associated antigen-1 (LFA-1) on in vivo natural killer cells. Eur J Immunol. 1988 Dec;18(12):2077–2080. doi: 10.1002/eji.1830181231. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Ohteki T., Abo T., Seki S., Kobata T., Yagita H., Okumura K., Kumagai K. Predominant appearance of gamma/delta T lymphocytes in the liver of mice after birth. Eur J Immunol. 1991 Jul;21(7):1733–1740. doi: 10.1002/eji.1830210722. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Lew A. M., Maloy W. L., Weston M. A., Bluestone J. A., Schwartz R. H., Coligan J. E., Kruisbeek A. M. Thymus-dependent and thymus-independent developmental pathways for peripheral T cell receptor-gamma delta-bearing lymphocytes. J Immunol. 1988 Jun 15;140(12):4091–4096. [PubMed] [Google Scholar]
- Preffer F. I., Kim C. W., Fischer K. H., Sabga E. M., Kradin R. L., Colvin R. B. Identification of pre-T cells in human peripheral blood. Extrathymic differentiation of CD7+CD3- cells into CD3+ gamma/delta+ or alpha/beta+ T cells. J Exp Med. 1989 Jul 1;170(1):177–190. doi: 10.1084/jem.170.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B. Characterization of V beta-bearing cells in athymic (nu/nu) mice suggests an extrathymic pathway for T cell differentiation. Eur J Immunol. 1990 Apr;20(4):919–925. doi: 10.1002/eji.1830200430. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991 Feb 1;173(2):483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Steinberg A. D., Law L. D., Talal N. The role of NZB-NZW F1 thymus in experimental tolerance and auto-immunity. Arthritis Rheum. 1970 Jul-Aug;13(4):369–377. doi: 10.1002/art.1780130402. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Tsudo M., Karasuyama H., Kitamura F., Kono T., Hatakeyama M., Taniguchi T., Miyasaka M. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol. 1991 Oct 1;147(7):2222–2228. [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992 Feb 5;146(2):145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- Wiltrout R. H., Pilaro A. M., Gruys M. E., Talmadge J. E., Longo D. L., Ortaldo J. R., Reynolds C. W. Augmentation of mouse liver-associated natural killer activity by biologic response modifiers occurs largely via rapid recruitment of large granular lymphocytes from the bone marrow. J Immunol. 1989 Jul 1;143(1):372–378. [PubMed] [Google Scholar]
- Yagita H., Nakata M., Azuma A., Nitta T., Takeshita T., Sugamura K., Okumura K. Activation of peripheral blood T cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Oct 1;170(4):1445–1450. doi: 10.1084/jem.170.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Reis M. D., Mak T. W. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986 Dec 4;324(6096):482–485. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]