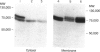
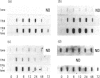
Abstract
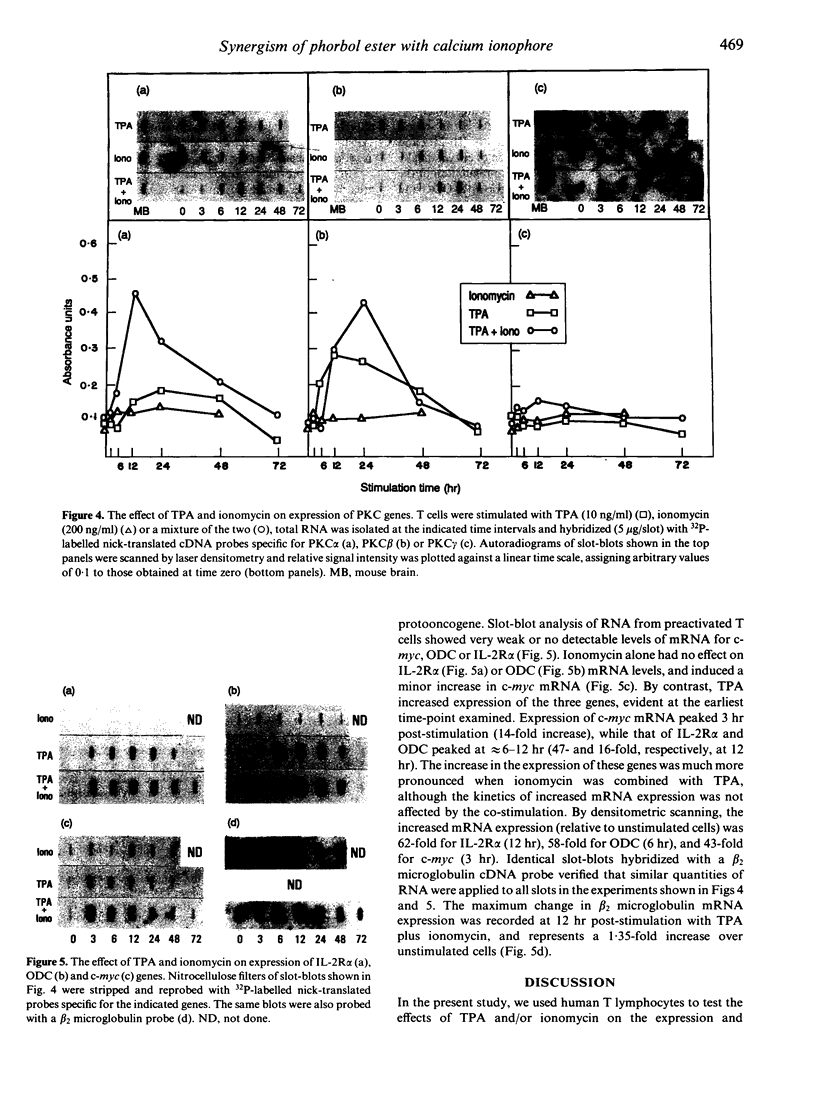
Studies described herein were designed to examine the effects of 12-O-tetradecanoyl phorbol-13-acetate (TPA), and a Ca2+ ionophore (ionomycin), singly or in combination, on the activation and expression of the Ca(2+)-dependent protein kinase C (PKC) isoenzymes (alpha, beta and gamma) at the protein and messenger RNA (mRNA) levels in T cells. These two agents induce the activation and proliferation of T lymphocytes by mimicking the action of inositol phospholipid-derived second messengers normally generated by triggering of the antigen-specific T-cell receptor (TcR)/CD3 complex. TPA-induced T-cell proliferation, expression of interleukin-2 receptor-alpha subunit (IL-2R alpha) and transferrin receptor, CD3 down-regulation and, lastly, the cytosol-to-membrane PKC translocation (determined by an enzymatic assay or by immunoblotting with a cross-reactive anti-PKC peptide antibody) were all facilitated by ionomycin. Immunoblots with isoenzyme-specific anti-PKC monoclonal antibodies demonstrated expression of immunoreactive PKC alpha, PKC beta and PKC gamma proteins that were translocated to the membrane upon TPA plus ionomycin stimulation. Resting T cells expressed abundant levels of mRNA for PKC alpha and PKC beta, but very low levels (relative to brain) of PKC gamma. TPA increased by two- to threefold the expression of PKC beta, but not of PKC alpha or PKC gamma, mRNA within 12 hr of stimulation. Ionomycin synergized with TPA in increasing the expression of PKC alpha and PKC beta mRNA. The two agents also synergized in inducing expression of additional activation/growth-associated genes, namely the c-myc protooncogene, ornithine decarboxylase (ODC) and IL-2R alpha. Ionomycin alone was inactive (or marginally active) in all of these assays. The translocation of distinct Ca(2+)-dependent PKC isoenzymes to the membrane and the up-regulation of PKC alpha and beta mRNA suggest that at least these two isoenzymes are involved in discrete steps of the pathway leading to T-cell activation and proliferation. Moreover, the combined effects of TPA and ionomycin on T-cell function and cell-surface antigen expression appear to be due, at least in part, to their synergistic activation of distinct PKC isoenzyme(s).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Borner C., Filipuzzi I., Wartmann M., Eppenberger U., Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J Biol Chem. 1989 Aug 15;264(23):13902–13909. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Noguchi P. D., Cunningham R. E., Waldmann T. A., Greene W. C. Regulation of interleukin 2 receptor expression: effects of phorbol diester, phospholipase C, and reexposure to lectin or antigen. J Immunol. 1984 Dec;133(6):3054–3061. [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W. Association of protein kinase C activation with IL 2 receptor expression. J Immunol. 1986 Feb 15;136(4):1266–1273. [PubMed] [Google Scholar]
- Hidaka H., Tanaka T., Onoda K., Hagiwara M., Watanabe M., Ohta H., Ito Y., Tsurudome M., Yoshida T. Cell type-specific expression of protein kinase C isozymes in the rabbit cerebellum. J Biol Chem. 1988 Apr 5;263(10):4523–4526. [PubMed] [Google Scholar]
- Housey G. M., O'Brian C. A., Johnson M. D., Kirschmeier P., Weinstein I. B. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1065–1069. doi: 10.1073/pnas.84.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Cunha-Melo J. R., Beaven M. A., Huang K. P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989 Mar 5;264(7):4238–4243. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N., Altman A. Human T lymphocyte activation by tumor promoters: role of protein kinase C. J Immunol. 1987 May 15;138(10):3100–3107. [PubMed] [Google Scholar]
- Isakov N., Altman A. Tumor promoters in conjunction with calcium ionophores mimic antigenic stimulation by reactivation of alloantigen-primed murine T lymphocytes. J Immunol. 1985 Dec;135(6):3674–3680. [PubMed] [Google Scholar]
- Isakov N., Bleackley R. C., Shaw J., Altman A. The tumor promoter teleocidin induces IL 2 receptor expression and IL 2-independent proliferation of human peripheral blood T cells. J Immunol. 1985 Oct;135(4):2343–2350. [PubMed] [Google Scholar]
- Isakov N., McMahon P., Altman A. Selective post-transcriptional down-regulation of protein kinase C isoenzymes in leukemic T cells chronically treated with phorbol ester. J Biol Chem. 1990 Feb 5;265(4):2091–2097. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Komada F., Nishikawa M., Uemura Y., Morita K., Hidaka H., Shirakawa S. Expression of three major protein kinase C isozymes in various types of human leukemic cells. Cancer Res. 1991 Aug 15;51(16):4271–4278. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Krug E., Biemann H. P., Tashjian A. H., Jr Evidence for increased synthesis as well as increased degradation of protein kinase C after treatment of human osteosarcoma cells with phorbol ester. J Biol Chem. 1987 Aug 25;262(24):11852–11856. [PubMed] [Google Scholar]
- Ledbetter J. A., Gentry L. E., June C. H., Rabinovitch P. S., Purchio A. F. Stimulation of T cells through the CD3/T-cell receptor complex: role of cytoplasmic calcium, protein kinase C translocation, and phosphorylation of pp60c-src in the activation pathway. Mol Cell Biol. 1987 Feb;7(2):650–656. doi: 10.1128/mcb.7.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Rothenberg E. V. Early precursor thymocytes can produce interleukin 2 upon stimulation with calcium ionophore and phorbol ester. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1862–1866. doi: 10.1073/pnas.83.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger B., Weiss A., Imboden J., Laing T., Stobo J. D. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987 Oct 15;139(8):2755–2760. [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwine-Kennick R. L., McKeegan E. M., Johnson M. D., Morin M. J. Phorbol diester-induced alterations in the expression of protein kinase C isozymes and their mRNAs. Analysis in wild-type and phorbol diester-resistant HL-60 cell clones. J Biol Chem. 1991 Aug 15;266(23):15135–15143. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Konno Y., Imajoh S., Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988 Jun 3;53(5):731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Imajoh S., Suzuki K., Inagaki M., Yokokura H., Sakoh T., Hidaka H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;325(7000):161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Scholz W., Altman A. Synergistic induction of interleukin 2 receptor (TAC) expression on YT cells by interleukin 1 or tumor necrosis factor alpha in combination with cAMP inducing agents. Cell Signal. 1989;1(4):367–375. doi: 10.1016/0898-6568(89)90055-7. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]