Abstract
To clarify the mechanism by which progenitor T (pro-T) cells recognize and enter the thymus, an attempt was made to produce haematopoietic cell lines by the fusion of BALB/c nude mouse bone marrow or foetal liver cells (gestation 14 and 15 days) with AKR thymoma BW5147, thereby immortalizing cells with potency to colonize the thymus, a characteristic of pro-T cells rarely found in adult bone marrow or foetal liver. The hybridomas thus produced were classified according to the phenotype of surface markers, T-cell receptor (TcR) gene configuration and expression. All hybridomas were negative in the surface expression of T-cell markers such as TcR alpha beta, TcR gamma delta, CD3, CD4 and CD8. They had TcR beta-, gamma- and delta-genes, each with a different status with respect to configuration and transcription. Some possessed partially rearranged TcR genes and others expressed immature TcR mRNA. The cell lines were examined for their capacity to colonize the thymus following intravenous injection into recipient mice. It was found that the cells with capacity of colonizing the thymus expressed immature TcR delta mRNA, while the cell lines lacking TcR delta-genes did not home to the thymus. These findings imply that the potency for migrating to thymus is closely associated with the particular stage of prethymic cell differentiation which could be estimated by the analysis of TcR genes, and that some cell lines with the expression of TcR delta-gene mRNA and the ability to colonize the thymus are derived from pro-T cells.
Full text
PDF

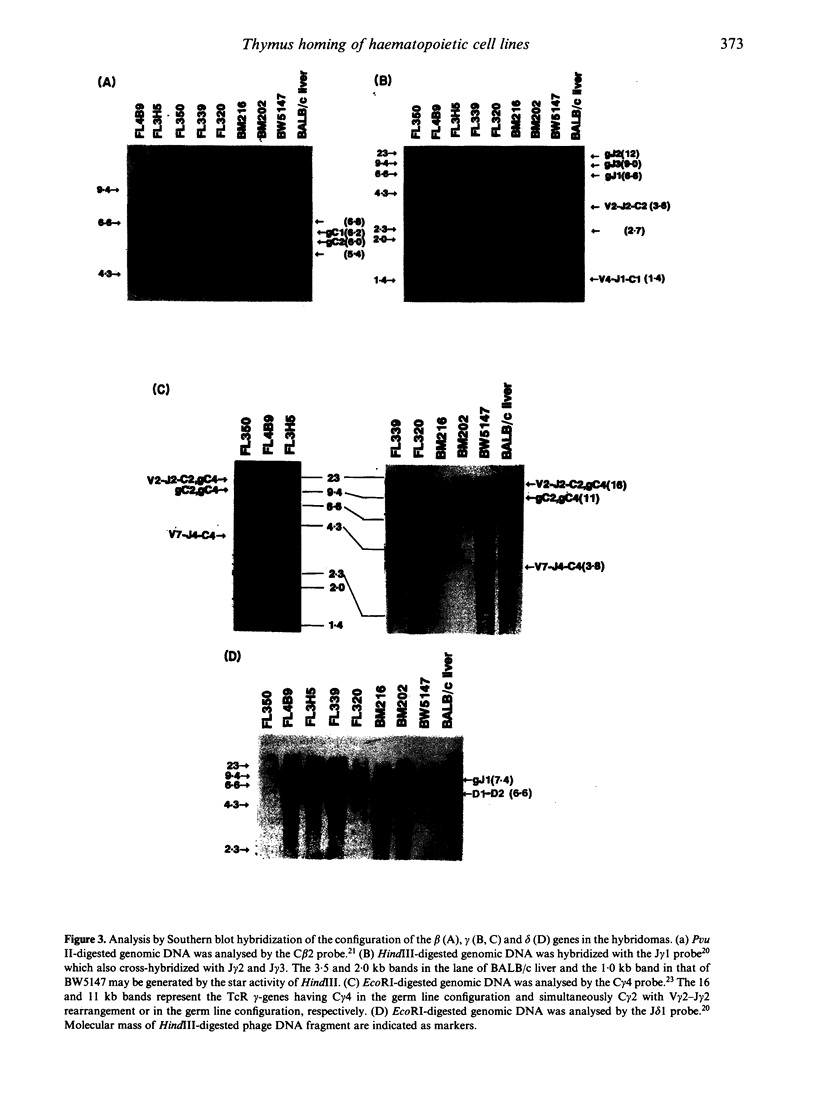
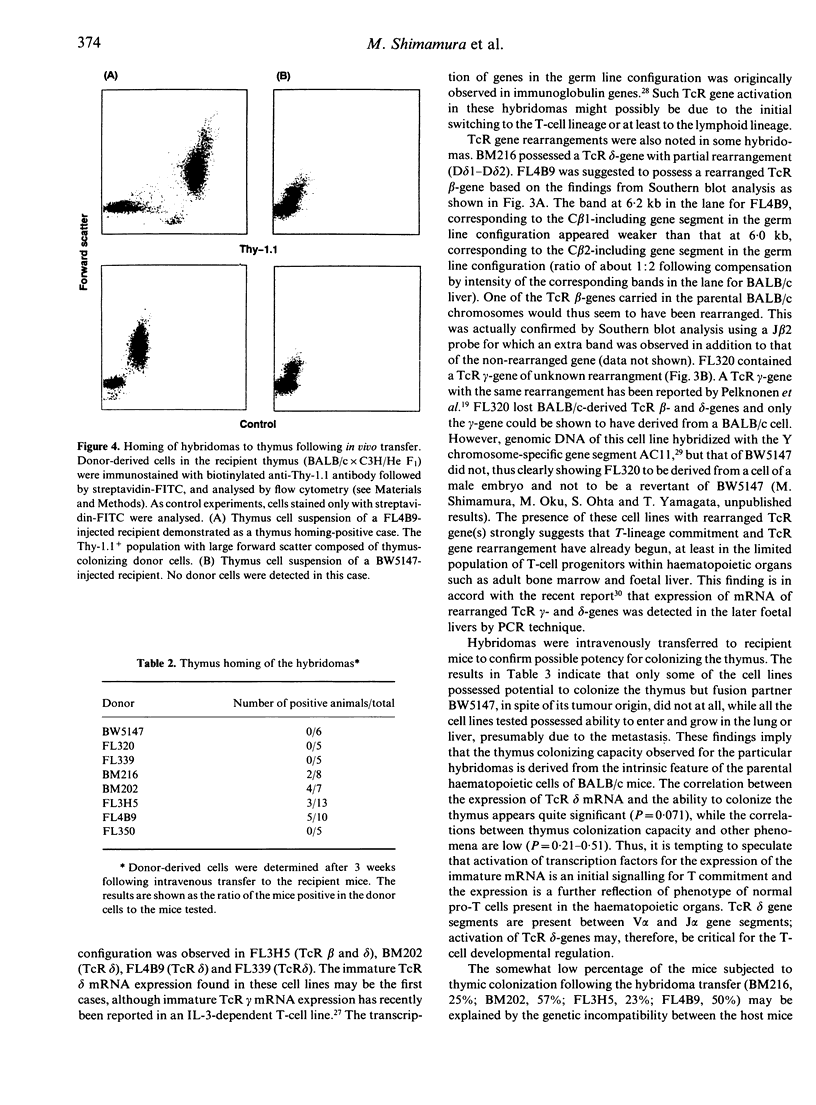
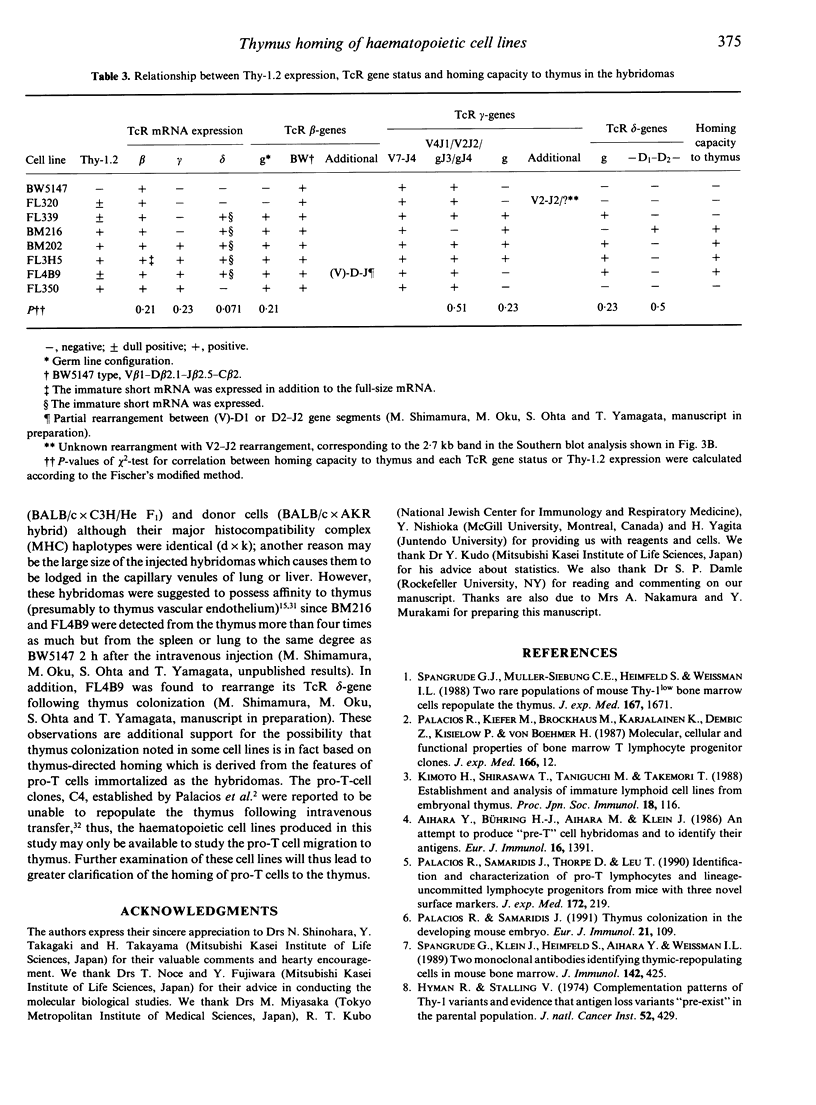

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aihara Y., Bühring H. J., Aihara M., Klein J. An attempt to produce "pre-T" cell hybridomas and to identify their antigens. Eur J Immunol. 1986 Nov;16(11):1391–1399. doi: 10.1002/eji.1830161113. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Calame K., Eaton S. Transcriptional controlling elements in the immunoglobulin and T cell receptor loci. Adv Immunol. 1988;43:235–275. doi: 10.1016/s0065-2776(08)60367-3. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E. G., Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Kyes S., Pao W., Hayday A. Influence of site of expression on the fetal gamma delta T-cell receptor repertoire. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7830–7833. doi: 10.1073/pnas.88.17.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Lamothe E. Isolation and characterization of a mouse Y chromosomal repetitive sequence. Genetics. 1986 Jun;113(2):417–432. doi: 10.1093/genetics/113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Kiefer M., Brockhaus M., Karjalainen K., Dembić Z., Kisielow P., von Boehmer H. Molecular, cellular, and functional properties of bone marrow T lymphocyte progenitor clones. J Exp Med. 1987 Jul 1;166(1):12–32. doi: 10.1084/jem.166.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Samaridis J., Thorpe D., Leu T. Identification and characterization of pro-T lymphocytes and lineage-uncommitted lymphocyte precursors from mice with three novel surface markers. J Exp Med. 1990 Jul 1;172(1):219–230. doi: 10.1084/jem.172.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Samaridis J. Thymus colonization in the developing mouse embryo. Eur J Immunol. 1991 Jan;21(1):109–113. doi: 10.1002/eji.1830210117. [DOI] [PubMed] [Google Scholar]
- Pelkonen J., Traunecker A., Karjalainen K. A new mouse TCR V gamma gene that shows remarkable evolutionary conservation. EMBO J. 1987 Jul;6(7):1941–1944. doi: 10.1002/j.1460-2075.1987.tb02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Klein J., Heimfeld S., Aihara Y., Weissman I. L. Two monoclonal antibodies identify thymic-repopulating cells in mouse bone marrow. J Immunol. 1989 Jan 15;142(2):425–430. [PubMed] [Google Scholar]
- Spangrude G. J., Muller-Sieburg C. E., Heimfeld S., Weissman I. L. Two rare populations of mouse Thy-1lo bone marrow cells repopulate the thymus. J Exp Med. 1988 May 1;167(5):1671–1683. doi: 10.1084/jem.167.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., Nakanishi N., Ishida I., Kanagawa O., Tonegawa S. T cell receptor-gamma and -delta genes preferentially utilized by adult thymocytes for the surface expression. J Immunol. 1989 Mar 15;142(6):2112–2121. [PubMed] [Google Scholar]
- Weinstein Y., Morishita K., Cleveland J. L., Ihle J. N. Interleukin 3 (IL-3) induces transcription from nonrearranged T cell receptor gamma loci in IL-3-dependent cell lines. J Exp Med. 1989 Jun 1;169(6):2059–2071. doi: 10.1084/jem.169.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]