Abstract
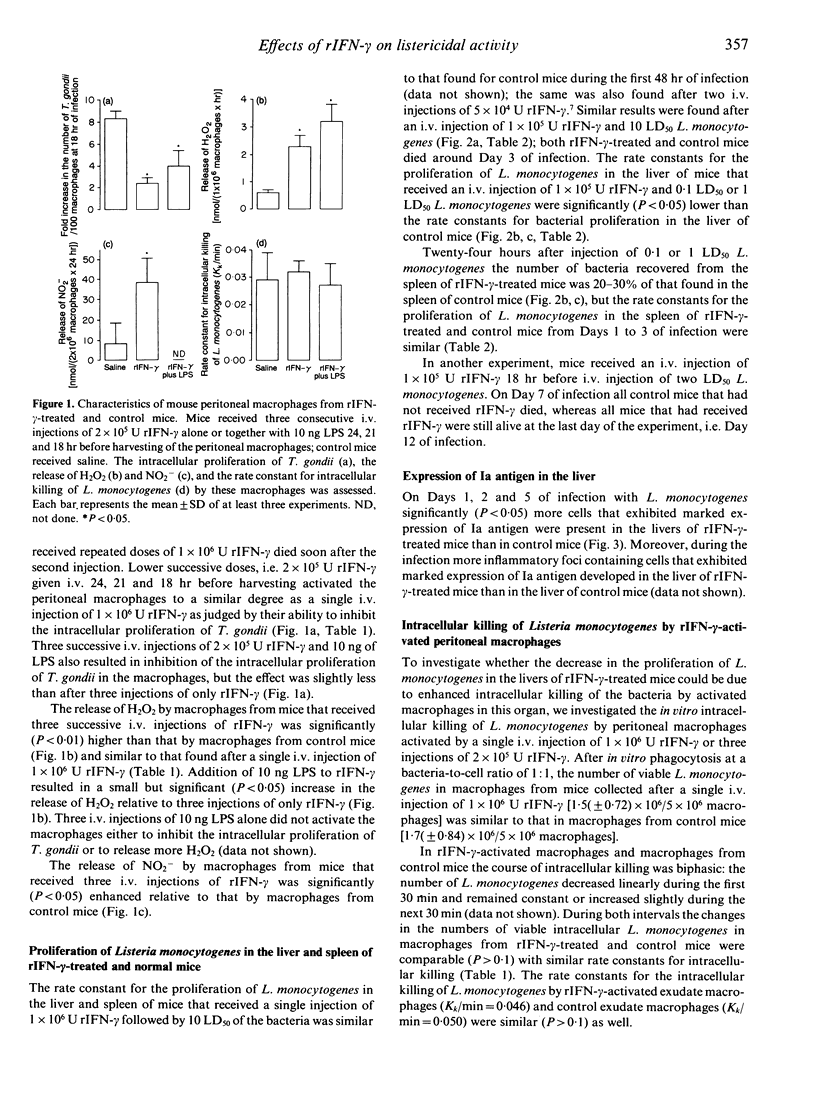
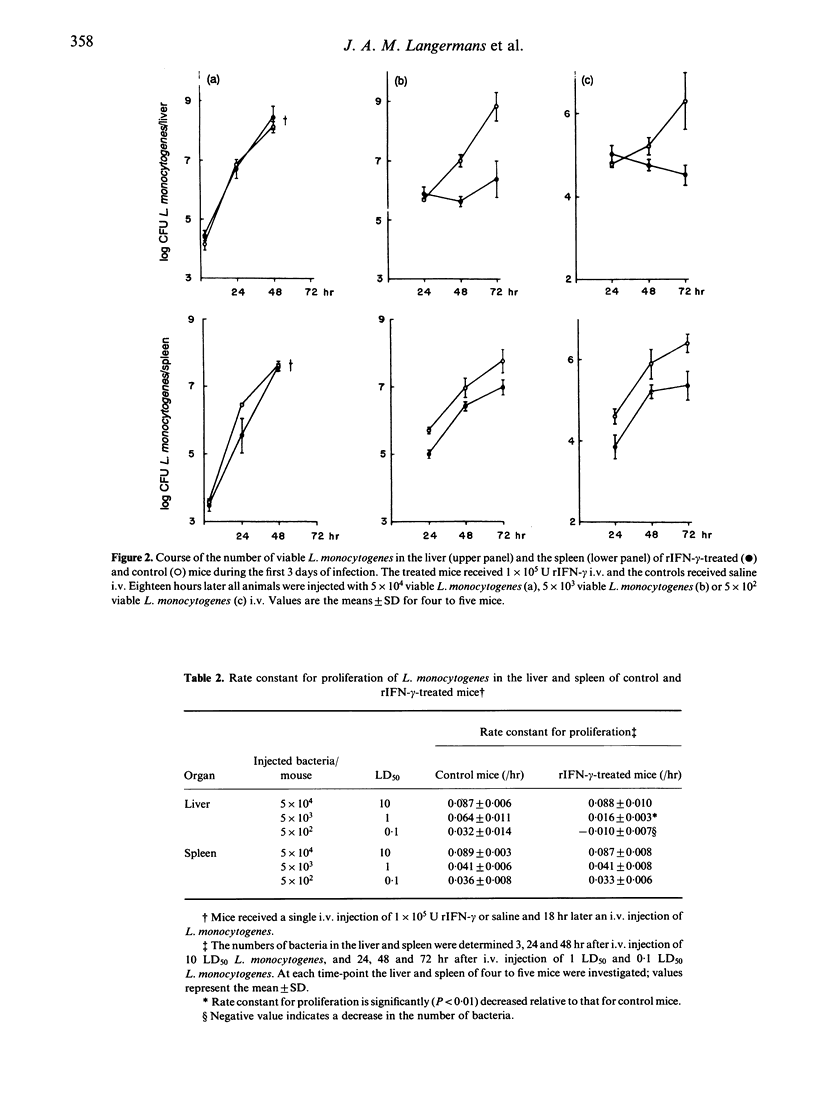
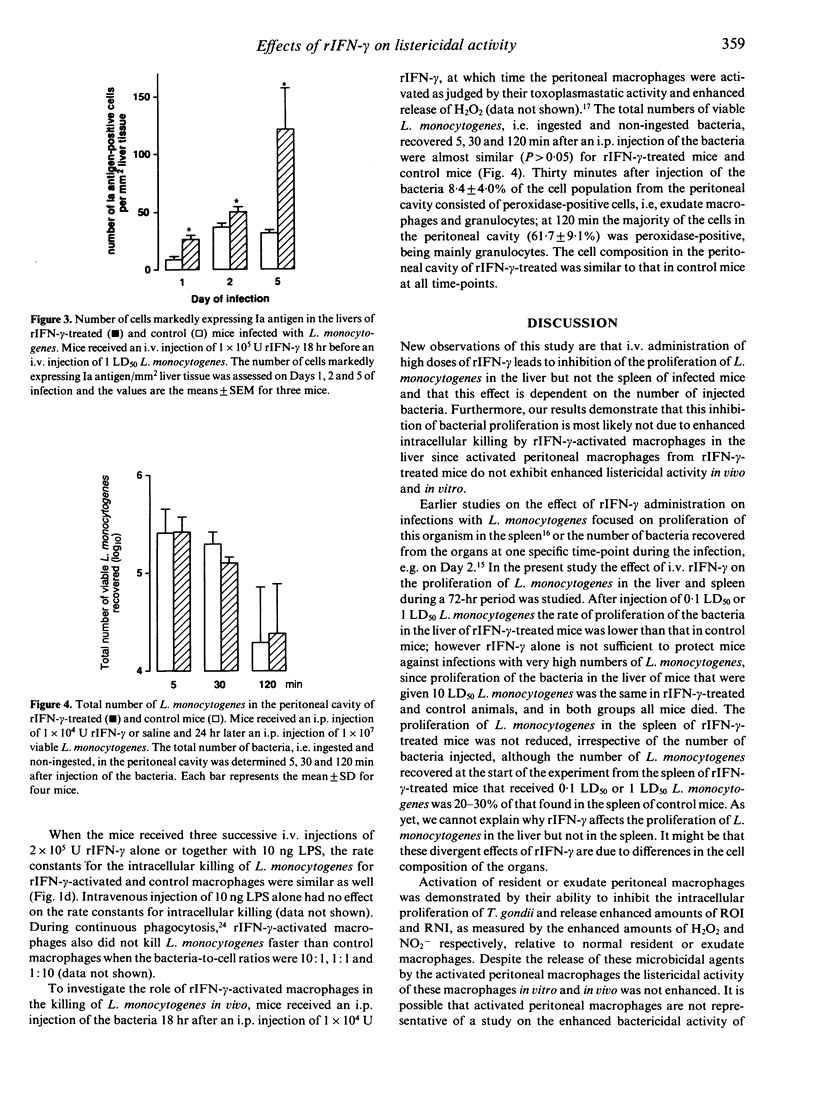
In the present study the effects of intravenous administration of recombinant interferon-gamma (IFN-gamma) on both the proliferation of Listeria monocytogenes in the liver and spleen of mice and the listericidal activity of their peritoneal macrophages were investigated. A single intravenous injection of 1 x 10(6) U or three injections of 2 x 10(5) U recombinant IFN-gamma (rIFN-gamma) induced optimal activation of resident and exudate peritoneal macrophages, as judged by their ability to inhibit the intracellular proliferation of Toxoplasma gondii and their enhanced release of H2O2 and NO2-. The rate of intracellular killing of L. monocytogenes by the rIFN-gamma-activated resident and exudate macrophages was not higher than that by resident macrophages. Addition of 10 ng lipopolysaccharides (LPS) to the rIFN-gamma also did not enhance the bactericidal activity of the activated peritoneal macrophages. The decrease in the number of L. monocytogenes in the peritoneal cavity of mice that had received an i.p. injection of 1 x 10(4) U rIFN-gamma was similar to that in control mice. Intravenous administration of 1 x 10(5) rIFN-gamma activated cells in the liver, as indicated by the increased expression of Ia antigen, and reduced the rate of proliferation of L. monocytogenes in the liver relative to that in control mice when 0.1 LD50 or 1 LD50 L. monocytogenes were injected. However, when 10 LD50 L. monocytogenes were administered there was no effect on their proliferation. The number of L. monocytogenes found initially in the spleen of rIFN-gamma-treated mice was 20-30% of that in the spleen of control mice, but the rate of proliferation of L. monocytogenes was not reduced. These divergent results for the proliferation of L. monocytogenes in the liver, spleen and peritoneal cavity indicate that cells other than macrophages and/or as yet unknown local factors play an important role in the listericidal activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A., Canono B. P., Cook J. L. Mouse macrophages stimulated by recombinant gamma interferon to kill tumor cells are not bactericidal for the facultative intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 May;56(5):1371–1375. doi: 10.1128/iai.56.5.1371-1375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Gregory S. H., Barczynski L. K., Wing E. J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukoc Biol. 1992 Apr;51(4):421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kurtz R. S., Young K. M., Czuprynski C. J. Separate and combined effects of recombinant interleukin-1 alpha and gamma interferon on antibacterial resistance. Infect Immun. 1989 Feb;57(2):553–558. doi: 10.1128/iai.57.2.553-558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Mellouk S., Green S. J., Nacy C. A., Hoffman S. L. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991 Jun 1;146(11):3971–3976. [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P. H., Langermans J. A., van de Gevel J. S., van der Hulst M. B., van Furth R. Nitrite production by activated murine macrophages correlates with their toxoplasmastatic activity, Ia antigen expression, and production of H2O2. Immunobiology. 1991 Dec;184(1):93–105. doi: 10.1016/S0171-2985(11)80575-9. [DOI] [PubMed] [Google Scholar]
- Nibbering P. H., van der Heide G. A., van Furth R. Immunocytochemical analysis of cellular responses to BCG. Clin Exp Immunol. 1989 Jan;75(1):147–154. [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Schreiber R. D., Connelly P., Tilney L. G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989 Dec 1;170(6):2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Falk P., Lohmüller M., van der Meide P. H. Class II MHC antigens in the rat digestive system. Normal distribution and induced expression after interferon-gamma treatment in vivo. Immunology. 1989 Dec;68(4):507–513. [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Van Vliet E., Melis M., Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol. 1984 Jun;14(6):524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., van den Barselaar M. T., Sluiter W., Leijh P. C., van Furth R. Divergent changes in antimicrobial activity after immunologic activation of mouse peritoneal macrophages. J Immunol. 1987 Sep 1;139(5):1665–1672. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


