Abstract
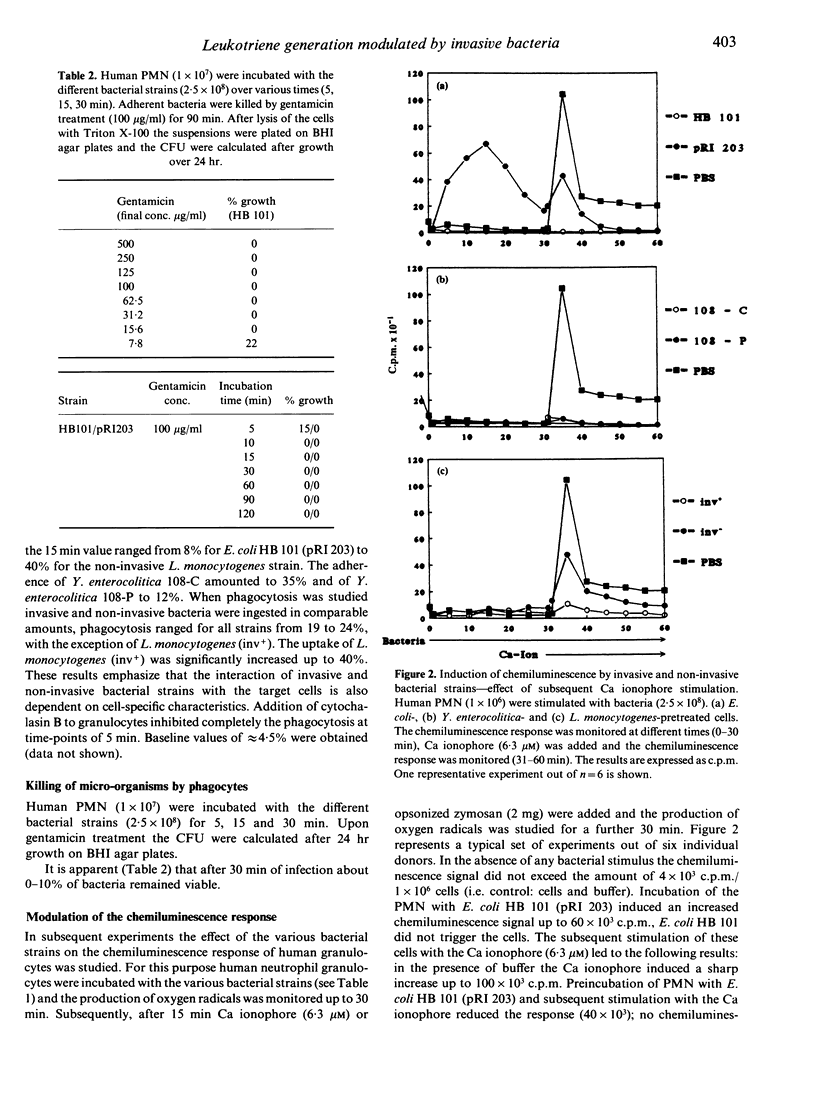
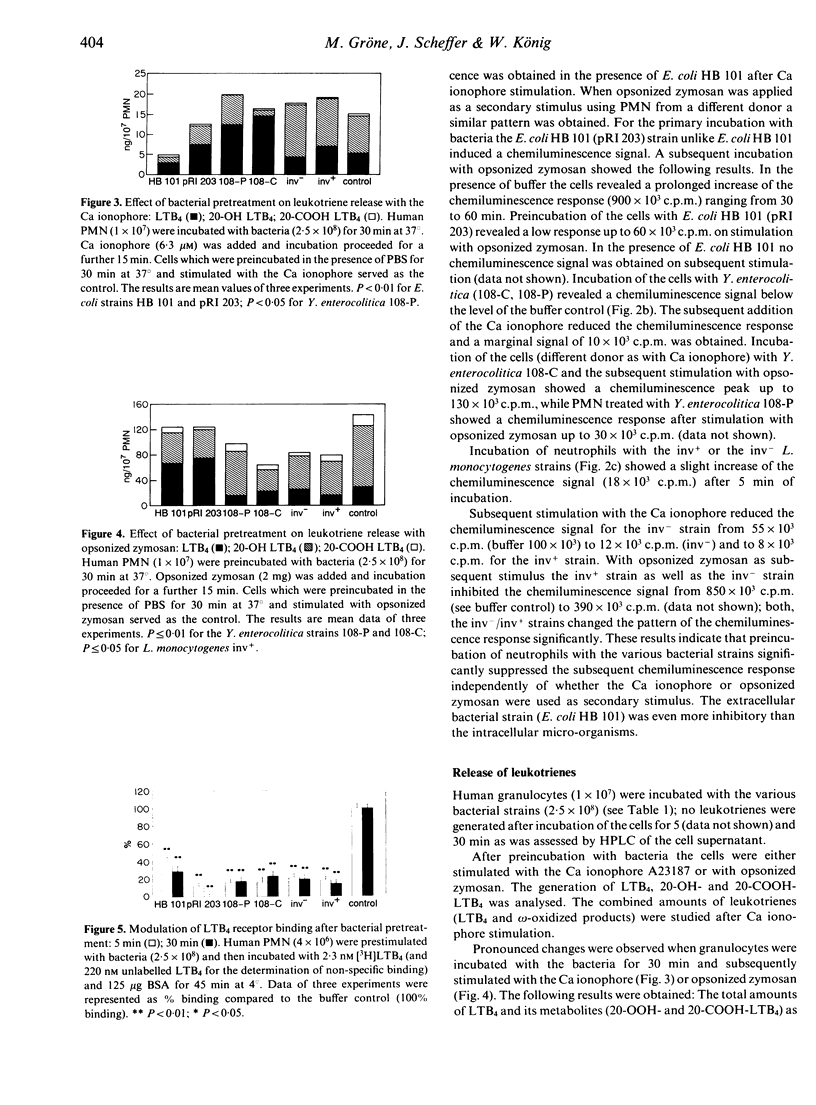
The effect of invasive bacteria on the release of proinflammatory mediators (oxygen radicals, leukotriene release) from human polymorphonuclear neutrophils was studied. Bacterial stimuli were used including genetically cloned invasive Yersinia enterocolitica strains 108-P (bearing the phagocytosis-resistance plasmid) and 108-C (plasmidless variant), Listeria monocytogenes [SLCC 5779 (inv-) and NCTC 7973 (inv+)] as well as an Escherichia coli K 12 strain (pRI 203) in which the inv gene of Y. pseudotuberculosis was cloned. When human polymorphonuclear granulocytes were studied as target cells the inv+ as well as the inv- strains were phagocytosed to a comparable amount with the exception of the L. monocytogenes strain (inv+). Among the invasive strains E. coli HB 101 (pRI 203) was the most active to trigger polymorphonuclear leucocytes (PMN) for oxygen radical production. Preincubation of the cells with bacteria and subsequent stimulation with the Ca ionophore A23187 or opsonized zymosan suppressed the chemiluminescence response to a different degree. The various bacterial strains did not induce leukotriene release from endogenous arachidonic acid. Subsequent stimulation of the infected cells with Ca ionophore or opsonized zymosan led to an altered pattern of the combined amounts of leukotriene B4 (LTB4), 20-OH- and 20-COOH-LTB4 as well as the ratio of LTB4 versus 20-OH and 20-COOH-LTB4. Infection of the cells also reduced strain dependently the number of LTB4-receptor sites. Our data suggest that bacterial uptake modulates the inflammatory response of granulocytes (e.g. chemiluminescence response, leukotriene generation).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann U., Scheffer J., Köller M., Schönfeld W., Erbs G., Müller F. E., König W. Induction of inflammatory mediators (histamine and leukotrienes) from rat peritoneal mast cells and human granulocytes by Pseudomonas aeruginosa strains from burn patients. Infect Immun. 1989 Jul;57(7):2187–2195. doi: 10.1128/iai.57.7.2187-2195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., König W., Pfeiffer P., Rauschen I., Theobald K., Thelestam M., Alouf J. E. Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun. 1985 Dec;50(3):844–851. doi: 10.1128/iai.50.3.844-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., König W., Spur B., Crea A., Galanos C. Generation of slow-reacting substance (leukotrienes) by endotoxin and lipid A from human polymorphonuclear granulocytes. Immunology. 1984 Oct;53(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Brom J., König W. Studies on the uptake, binding and metabolism of leukotriene B4 by human neutrophils. Immunology. 1989 Dec;68(4):479–485. [PMC free article] [PubMed] [Google Scholar]
- Brom J., Schönfeld W., König W. Metabolism of leukotriene B4 by activated human polymorphonuclear granulocytes. Immunology. 1988 Jul;64(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1600–1604. [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Gross U., Schmidt N., Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986 Nov;54(2):561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985 Aug;22(2):168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S., Leimeister-Wächter M., Chakraborty T., Lottspeich F., Goebel W. The gene coding for protein p60 of Listeria monocytogenes and its use as a specific probe for Listeria monocytogenes. Infect Immun. 1990 Jun;58(6):1943–1950. doi: 10.1128/iai.58.6.1943-1950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., Schönfeld W., Scheffer J., König W. Signal transduction in human platelets and inflammatory mediator release induced by genetically cloned hemolysin-positive and -negative Escherichia coli strains. Infect Immun. 1990 Jun;58(6):1591–1599. doi: 10.1128/iai.58.6.1591-1599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulf M., König W. Modulation of leukotriene release from human polymorphonuclear leucocytes by PMA and arachidonic acid. Immunology. 1988 May;64(1):51–59. [PMC free article] [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985 May;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Devenish J. A. Relationship of HeLa cell infectivity to biochemical, serological, and virulence characteristics of Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):497–506. doi: 10.1128/iai.35.2.497-506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberman R. J., Harper T. W., Murphy R. C., Austen K. F. Identification and functional characterization of leukotriene B4 20-hydroxylase of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2292–2295. doi: 10.1073/pnas.82.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüning M., Raulf M., König W. Localization of 5-lipoxygenase within human polymorphonuclear leukocytes. Biochem Pharmacol. 1985 Nov 15;34(22):3943–3950. doi: 10.1016/0006-2952(85)90370-3. [DOI] [PubMed] [Google Scholar]
- Une T., Zen-Yoji H., Maruyama T., Yanagawa Y. Correlation between epithelial cell infectivity in vitro and O-antigen groups of Yersinia enterocolitica. Microbiol Immunol. 1977;21(12):727–729. doi: 10.1111/j.1348-0421.1977.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Hoover C. S. Rickettsial effects on leukotriene and prostaglandin secretion by mouse polymorphonuclear leukocytes. Infect Immun. 1991 Jan;59(1):351–356. doi: 10.1128/iai.59.1.351-356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


