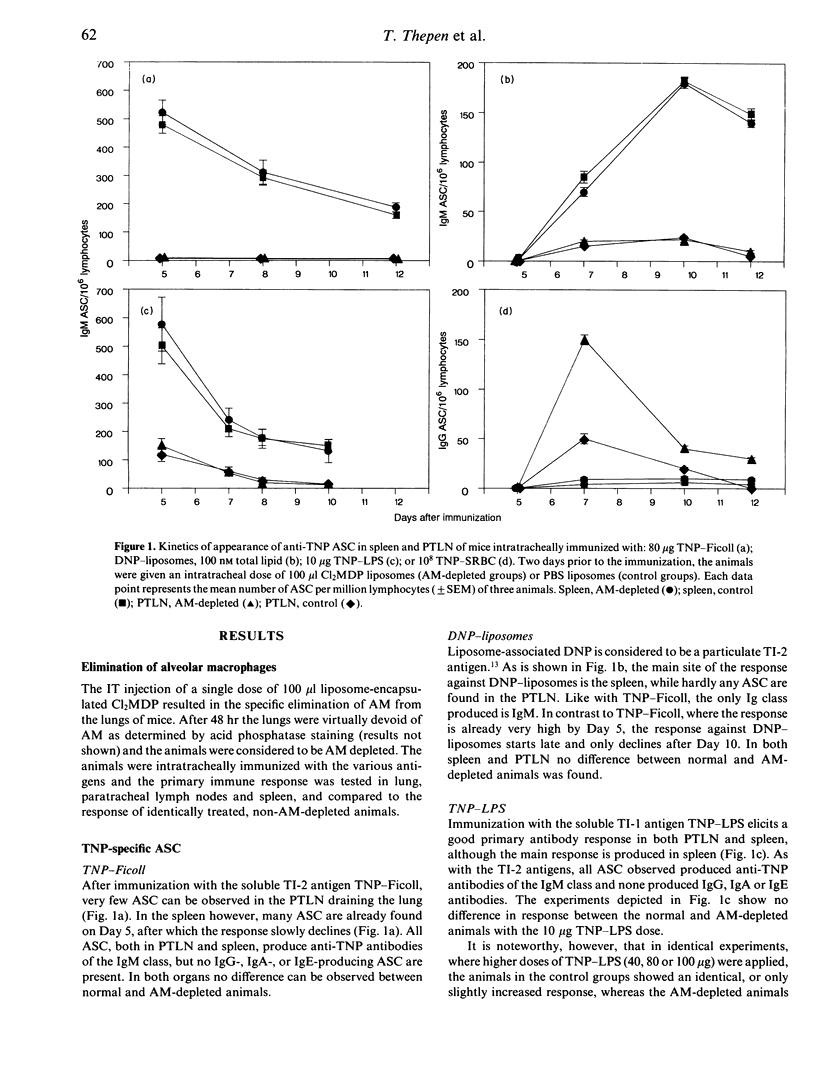
Abstract
The role of alveolar macrophages in the pulmonary immune response against various antigens was studied after elimination of alveolar macrophages by intratracheal administration of liposome-encapsulated dichloromethylene diphosphanate. When the responses against T-cell-independent type 1 and type 2 antigens were compared, it was found that elimination of alveolar macrophages had no effect on T-cell-independent antigens. Intratracheal antigen administration resulted in low lung associated, local responses, although some response was observed in the spleen. In contrast, elimination of alveolar macrophages resulted in an increase in local pulmonary immune response against T-cell-dependent antigens. We conclude from these experiments that alveolar macrophages play an important role in controlling the local pulmonary immune response against T-cell-dependent antigens by down-regulation of local T-cell populations. The alveolar macrophages do not down-regulate the response against intratracheally administered T-cell-independent antigens, although they are important in the protection against inflammatory damage caused by bacterial endotoxins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-trinitrophenyl antibody-forming cells in the murine spleen. Eur J Immunol. 1986 Mar;16(3):271–276. doi: 10.1002/eji.1830160311. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., van Rooijen N. Immunomodulation with liposomes: the immune response elicited by liposomes with entrapped dichloromethylene-diphosphonate and surface-associated antigen or hapten. Immunology. 1987 Apr;60(4):509–515. [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Van Rooijen N. TNP-enzyme conjugates for the detection of anti-TNP antibody producing cells in vivo. J Immunol Methods. 1984 Dec 14;75(1):181–188. doi: 10.1016/0022-1759(84)90237-0. [DOI] [PubMed] [Google Scholar]
- Goud S. N., Kaplan A. M., Subbarao B. Primary antibody responses to thymus-independent antigens in the lungs and hilar lymph nodes of mice. Infect Immun. 1990 Jul;58(7):2035–2041. doi: 10.1128/iai.58.7.2035-2041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud S. N., Muthusamy N., Subbarao B. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988 May 1;140(9):2925–2930. [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., McMenamin C. Defence against allergic sensitization in the healthy lung: the role of inhalation tolerance. Clin Exp Allergy. 1989 May;19(3):255–262. doi: 10.1111/j.1365-2222.1989.tb02380.x. [DOI] [PubMed] [Google Scholar]
- Inman J. K. Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J Immunol. 1975 Feb;114(2 Pt 1):704–709. [PubMed] [Google Scholar]
- Kaltreider H. B. Expression of immune mechanisms in the lung. Am Rev Respir Dis. 1976 Mar;113(3):347–379. doi: 10.1164/arrd.1976.113.3.347. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Naor D., Morecki S., Mitchell G. F. Differential induction of anti-trinitrophenyl plaque-forming cell responses to lightly and heavily conjugated trinitrophenylated heterologous and autologous erythrocytes in mice. Eur J Immunol. 1974 Apr;4(4):311–314. doi: 10.1002/eji.1830040415. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Thepen T., McMenamin C., Oliver J., Kraal G., Holt P. G. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur J Immunol. 1991 Nov;21(11):2845–2850. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- van Houte A. J., Snippe H., Willers J. M. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. I. Thymus independence of the antigen. Immunology. 1979 Jun;37(2):505–514. [PMC free article] [PubMed] [Google Scholar]


