Abstract
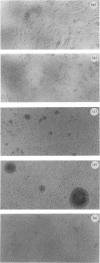
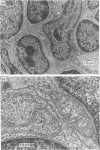
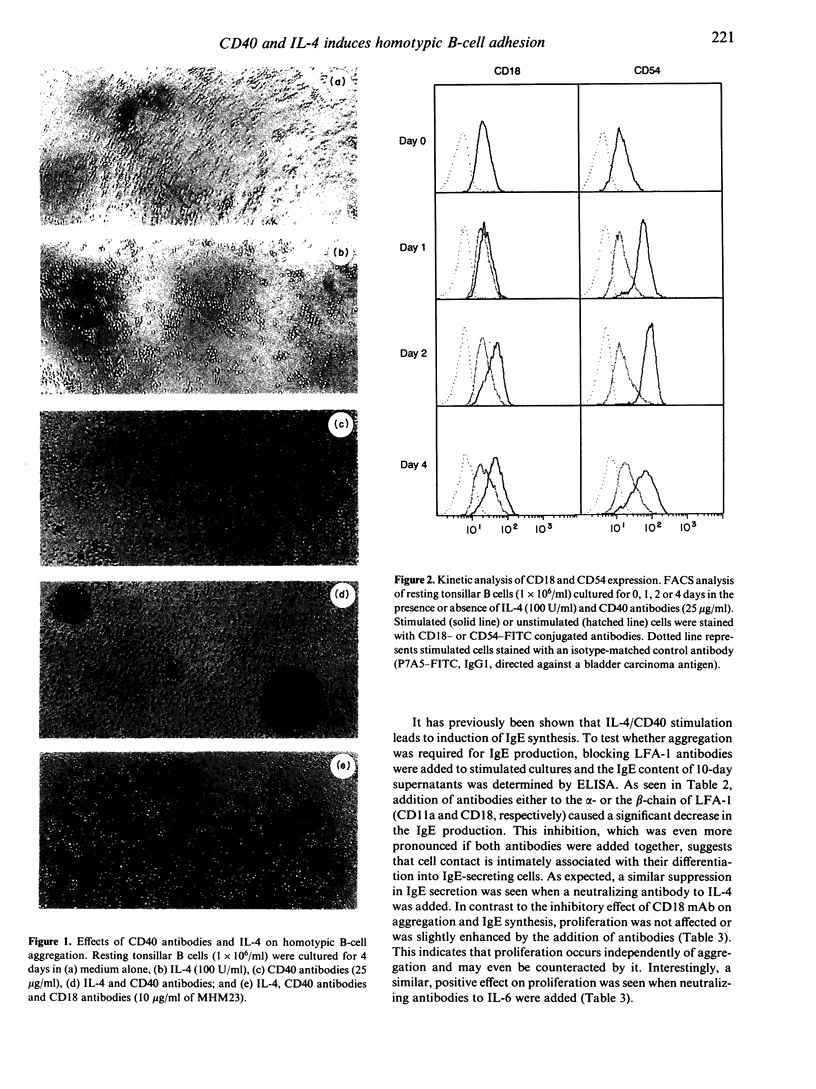
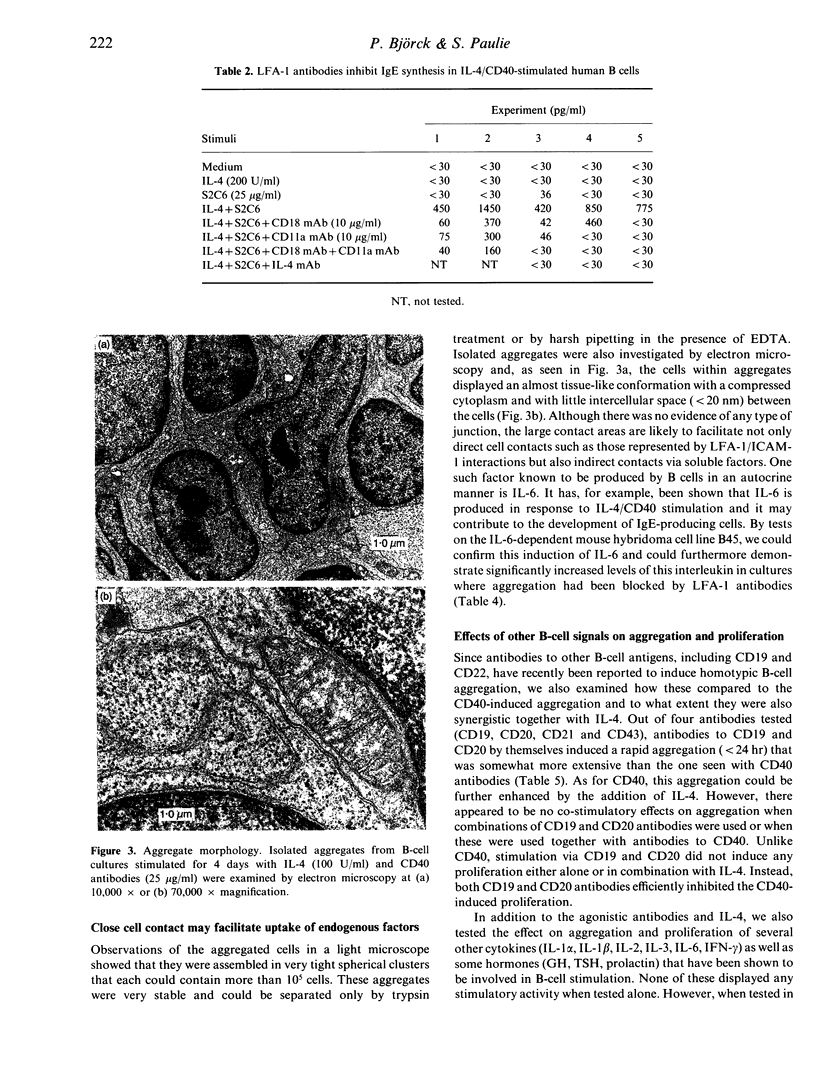
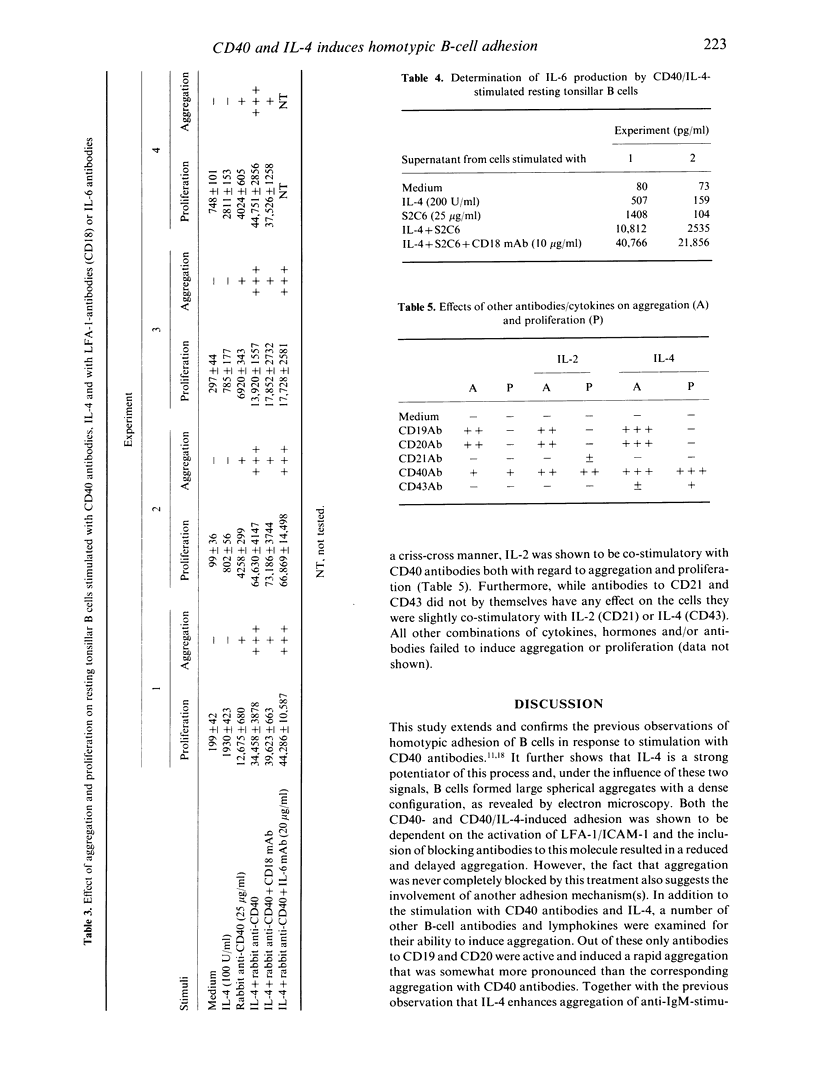
Antibodies to CD40 have been shown to induce homotypic aggregation of human resting B cells and B-cell lines via an LFA-1-dependent mechanism. We show here that interleukin-4 (IL-4) is a strong potentiator of this process and stimulation of tonsillar B cells for 4 days with IL-4 and CD40 antibodies resulted in the formation of large, dense aggregates. Also in this case, aggregation appeared to be chiefly dependent on the activation of LFA-1, although the small clusters of cells remaining after blocking with LFA-1 antibodies suggest the involvement of another adhesion system(s). When testing the relationship between aggregation and IgE synthesis, a known consequences of IL-4/CD40 stimulation, IgE levels were found to be significantly decreased in the presence of LFA-1 antibodies. In contrast to these observations, proliferation occurring in response to the IL-4/CD40 stimulation was not inhibitable by LFA-1 antibodies. Rather, in most cases, this was slightly enhanced, suggesting that aggregation may have a limiting effect on cell growth. Isolated aggregates, each of which could comprise more than 10(5) cells, were also examined by electron microscopy. This revealed a tissue-like structure of the aggregates with large contact areas and with minimal intercellular space between the adjacent cells. As the apparent inhibitory effect of aggregation on proliferation may reflect a negative autocrine signalling, which is enhanced by the close cell contact, we also tested the effect of neutralizing antibodies to IL-6, one of the factors known to be produced in the system. Such treatment did not affect aggregation but in most experiments enhanced proliferation. The results suggest that a possible effect of aggregation may be to enhance differentiation of cells and that this may also be associated with the difficulties in growing B cells in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Banchereau J., Rousset F. Growing human B lymphocytes in the CD40 system. Nature. 1991 Oct 17;353(6345):678–679. doi: 10.1038/353678a0. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Shu G., Clark E. A. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J Immunol. 1991 Mar 15;146(6):1722–1729. [PubMed] [Google Scholar]
- Björck P., Paulie S., Axelsson B. Interleukin-4-mediated aggregation of anti-IgM-stimulated human B cells: inhibition of aggregation but enhancement of proliferation by antibodies to LFA-1. Immunology. 1992 Jan;75(1):122–128. [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Clinchy B., Elenström C., Severinson E., Möller G. T and B cell collaboration: induction of motility in small, resting B cells by interleukin 4. Eur J Immunol. 1991 Jun;21(6):1445–1451. doi: 10.1002/eji.1830210618. [DOI] [PubMed] [Google Scholar]
- Gordon J., Millsum M. J., Guy G. R., Ledbetter J. A. Resting B lymphocytes can be triggered directly through the CDw40 (Bp50) antigen. A comparison with IL-4-mediated signaling. J Immunol. 1988 Mar 1;140(5):1425–1430. [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas G. S., Tedder T. F. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-1-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J Immunol. 1991 Dec 15;147(12):4094–4102. [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Paulie S., Rosén A., Ehlin-Henriksson B., Braesch-Andersen S., Jakobson E., Koho H., Perlmann P. The human B lymphocyte and carcinoma antigen, CDw40, is a phosphoprotein involved in growth signal transduction. J Immunol. 1989 Jan 15;142(2):590–595. [PubMed] [Google Scholar]
- Pircher H., Groscurth P., Baumhütter S., Aguet M., Zinkernagel R. M., Hengartner H. A monoclonal antibody against altered LFA-1 induces proliferation and lymphokine release of cloned T cells. Eur J Immunol. 1986 Feb;16(2):172–181. doi: 10.1002/eji.1830160212. [DOI] [PubMed] [Google Scholar]
- Schwartz-Albiez R., Dörken B., Monner D. A., Moldenhauer G. CD22 antigen: biosynthesis, glycosylation and surface expression of a B lymphocyte protein involved in B cell activation and adhesion. Int Immunol. 1991 Jul;3(7):623–633. doi: 10.1093/intimm/3.7.623. [DOI] [PubMed] [Google Scholar]
- Smeland E. B., Blomhoff H. K., Funderud S., Shalaby M. R., Espevik T. Interleukin 4 induces selective production of interleukin 6 from normal human B lymphocytes. J Exp Med. 1989 Oct 1;170(4):1463–1468. doi: 10.1084/jem.170.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Rigley K. P., Callard R. E. Activation of human B cells through the CD19 surface antigen results in homotypic adhesion by LFA-1-dependent and -independent mechanisms. Immunology. 1991 Jul;73(3):293–297. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Wikén M., Björck P., Axelsson B., Perlmann P. Induction of CD43 expression during activation and terminal differentiation of human B cells. Scand J Immunol. 1988 Oct;28(4):457–464. doi: 10.1111/j.1365-3083.1988.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Islam L. N. Recombinant IL-4 and IFN-gamma activate locomotor capacity in human B lymphocytes. Immunology. 1989 Jun;67(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A. R., Springer T. A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992 Jan 1;175(1):185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]