Abstract
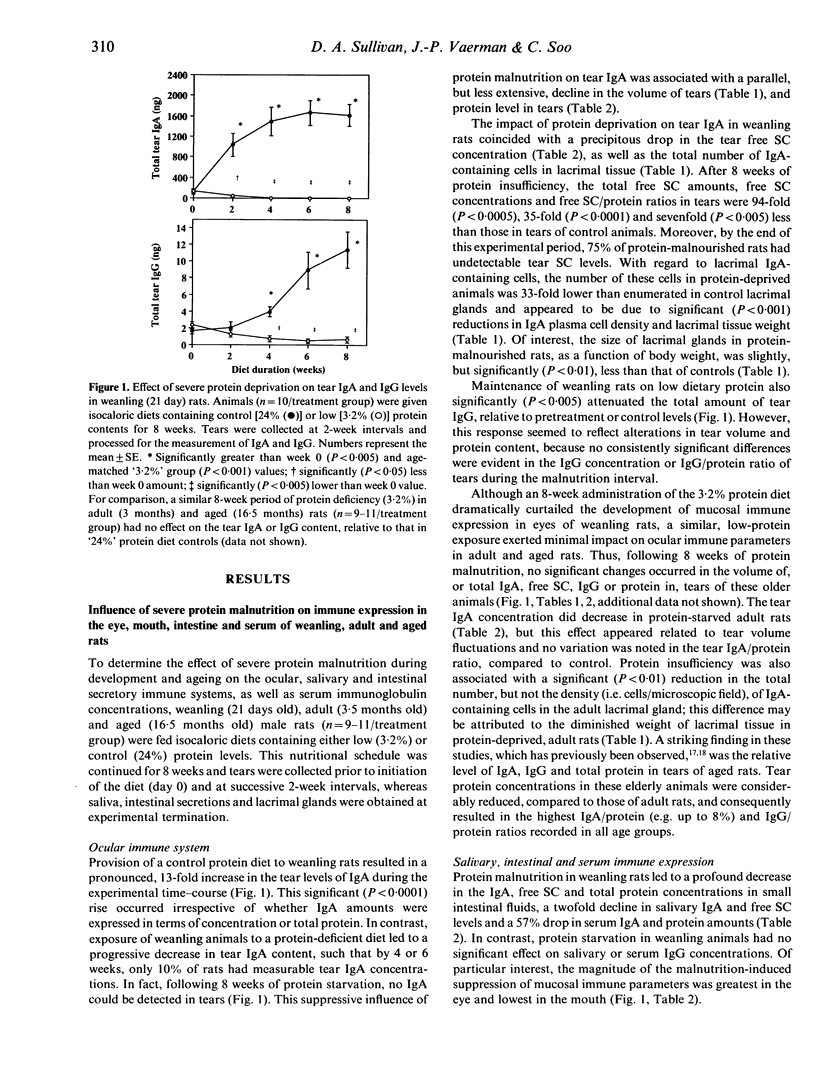
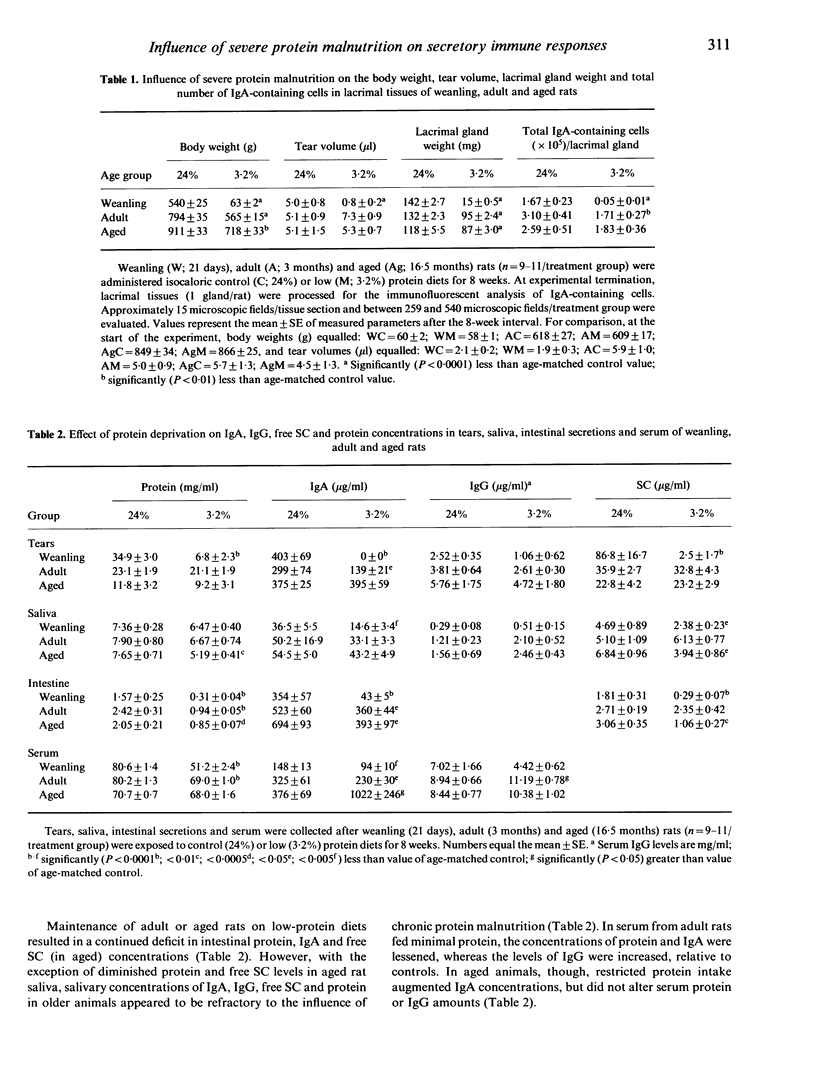
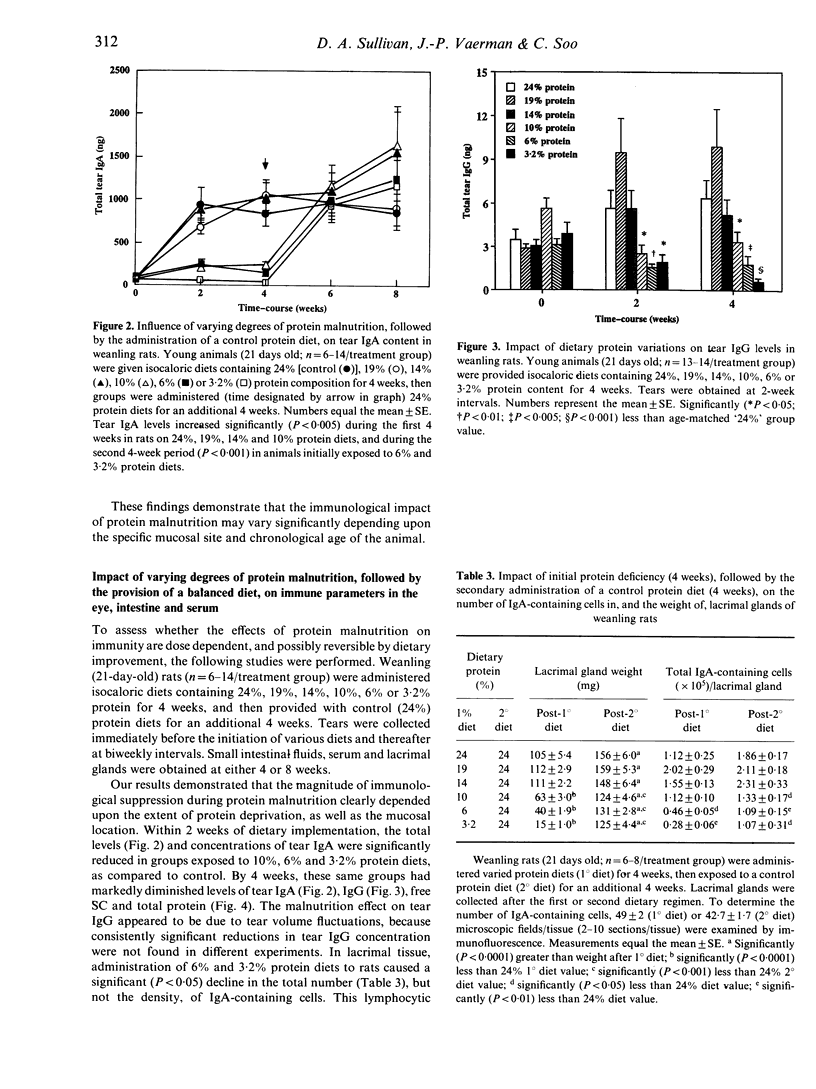
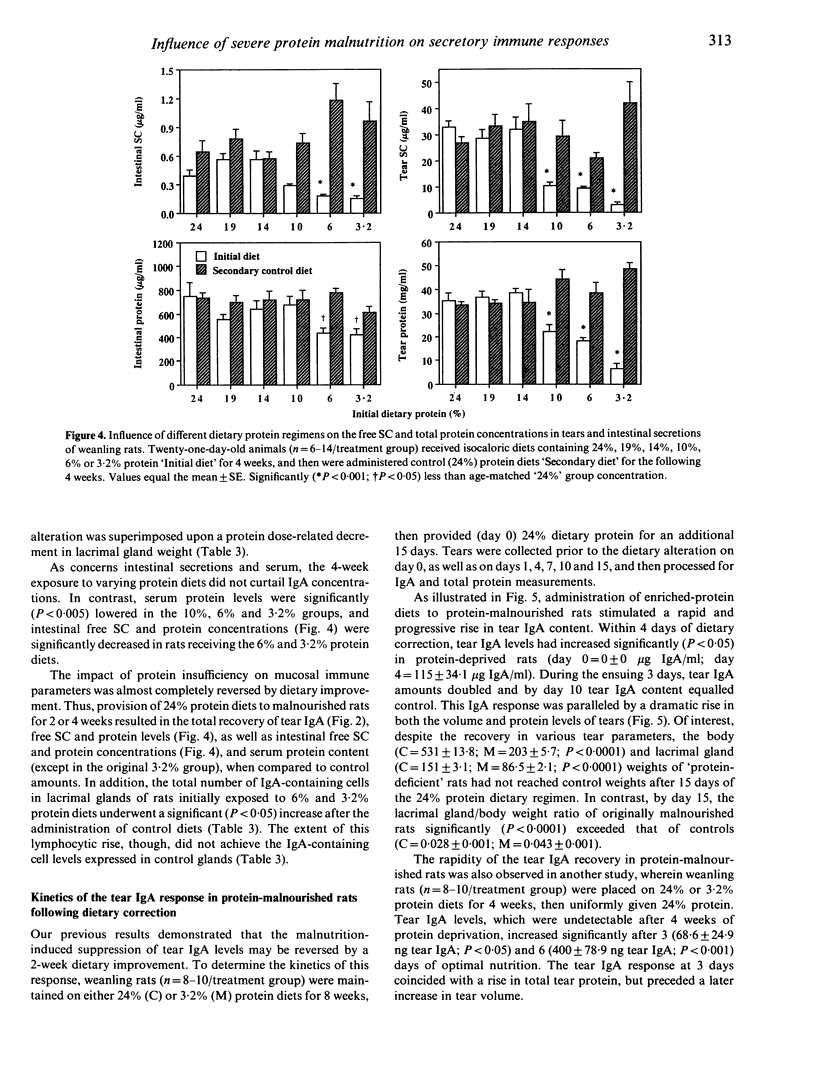
The objective of the present study was to examine and to compare the impact of severe protein malnutrition during development, adulthood and ageing on secretory immune expression in the eye, mouth and small intestine. In addition, we sought to determine whether potential abrogation of mucosal immunity by protein deprivation might be reversed by the administration of a balanced diet. Weanling, adult and aged rats were provided isocaloric diets containing 24% (control), 19%, 14%, 10%, 6% and/or 3.2% protein levels for defined periods and various immunological parameters were evaluated before, during and after the dietary regimen. Our results demonstrated the following. (1) Severe protein malnutrition (3.2%) dramatically suppressed the secretory immune system in eyes of weanling rats. After 8 weeks of protein insufficiency, tear IgA concentrations in young rats had undergone a precipitous decrease, such that IgA could not be detected in tears. This response was paralleled by a significant decline in the tear volume, tear secretory component (SC), IgG and total protein content, number of IgA-containing cells in lacrimal tissue, as well as the amounts of SC and/or IgA in saliva, intestinal secretions and serum. In contrast, the immunological effects of protein malnutrition in adult or aged animals varied considerably depending upon the specific mucosal site. (2) The influence of protein deprivation was dose dependent and reversible: maintenance of weanling rats on 10%, 6% or 3.2% protein diets interfered with the establishment of ocular and intestinal mucosal immunity, but later administration of optimal diets to these malnourished animals permitted a rapid immune recovery. (3) The impact of protein malnutrition on tear IgA levels in weanling animals, as shown by pair-feeding experiments, appeared to reflect primarily protein deficiency and not caloric restriction. Overall, these findings show that dietary protein plays a significant, site-specific role in the developmental expression of the secretory immune system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Gudmundsson O. G., Hann L. E., Keys C., Bloch K. J., Taubman M. A., Sullivan D. A. The immune response of the lacrimal gland to antigenic exposure. Curr Eye Res. 1987 Jul;6(7):921–927. doi: 10.3109/02713688709034860. [DOI] [PubMed] [Google Scholar]
- Altamirano G. A., Barranco-Acosta C., van Roost E., Vaerman J. P. Isolation and characterization of secretory IgA (sIgA) and free secretory component (FSC) from rat bile. Mol Immunol. 1980 Dec;17(12):1525–1537. doi: 10.1016/0161-5890(80)90178-9. [DOI] [PubMed] [Google Scholar]
- Barry W. S., Pierce N. F. Protein deprivation causes reversible impariment of mucosal immune response to cholera toxoid/toxin in rat gut. Nature. 1979 Sep 6;281(5726):64–65. doi: 10.1038/281064a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Nilssen D. E., Rognum T. O., Thrane P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991 Sep;20(3):397–439. [PubMed] [Google Scholar]
- Buts J. P., De Keyser N., Dive C. Intestinal development in the suckling rat: effect of insulin on the maturation of villus and crypt cell functions. Eur J Clin Invest. 1988 Aug;18(4):391–398. doi: 10.1111/j.1365-2362.1988.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Buts J. P., Delacroix D. L. Ontogenic changes in secretory component expression by villous and crypt cells of rat small intestine. Immunology. 1985 Jan;54(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Buts J. P., Vaerman J. P., Lescoat G. Ontogeny of the receptor for polymeric immunoglobulins in rat hepatocytes. Gastroenterology. 1992 Mar;102(3):949–955. doi: 10.1016/0016-5085(92)90181-w. [DOI] [PubMed] [Google Scholar]
- CARRIERE R. THE INFLUENCE OF THE THYROID GLAND ON POLYPLOID CELL FORMATION IN THE EXTERNAL ORBITAL GLAND OF THE RAT. Am J Anat. 1964 Jul;115:1–15. doi: 10.1002/aja.1001150102. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Mucosal immune responses in malnutrition. Ann N Y Acad Sci. 1983 Jun 30;409:345–352. doi: 10.1111/j.1749-6632.1983.tb26882.x. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Nutritional regulation of immunity and risk of infection in old age. Immunology. 1989 Jun;67(2):141–147. [PMC free article] [PubMed] [Google Scholar]
- Childers N. K., Bruce M. G., McGhee J. R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- Chua S. C., Jr, Leibel R. L., Hirsch J. Food deprivation and age modulate neuropeptide gene expression in the murine hypothalamus and adrenal gland. Brain Res Mol Brain Res. 1991 Jan;9(1-2):95–101. doi: 10.1016/0169-328x(91)90134-j. [DOI] [PubMed] [Google Scholar]
- Cruz J. R., Carlsson B., García B., Gebre-Medhin M., Hofvander Y., Urrutia J. J., Hanson L. A. Studies on human milk III. Secretory IgA quantity and antibody levels against Escherichia coli in colostrum and milk from underprivileged and privileged mothers. Pediatr Res. 1982 Apr;16(4 Pt 1):272–276. doi: 10.1203/00006450-198204000-00004. [DOI] [PubMed] [Google Scholar]
- Daniels C. K., Schmucker D. L., Bazin H., Jones A. L. Immunoglobulin A receptor of rat small intestinal enterocytes is unaffected by aging. Gastroenterology. 1988 Jun;94(6):1432–1440. doi: 10.1016/0016-5085(88)90683-x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980 Sep-Oct;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- Glass A. R., Swerdloff R. S. Nutritional influences on sexual maturation in the rat. Fed Proc. 1980 May 15;39(7):2360–2364. [PubMed] [Google Scholar]
- Glass R. I., Svennerholm A. M., Stoll B. J., Khan M. R., Huda S., Huq M. I., Holmgren J. Effects of undernutrition on infection with Vibrio cholerae O1 and on response to oral cholera vaccine. Pediatr Infect Dis J. 1989 Feb;8(2):105–109. [PubMed] [Google Scholar]
- Haq J. A., Szewczuk M. R. Differential effect of aging on B-cell immune responses to cholera toxin in the inductive and effector sites of the mucosal immune system. Infect Immun. 1991 Sep;59(9):3094–3100. doi: 10.1128/iai.59.9.3094-3100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hennart P. F., Brasseur D. J., Delogne-Desnoeck J. B., Dramaix M. M., Robyn C. E. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991 Jan;53(1):32–39. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Kiely J. Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig Dis Sci. 1989 Feb;34(2):175–184. doi: 10.1007/BF01536048. [DOI] [PubMed] [Google Scholar]
- Koster F., Pierce N. F. Effect of protein deprivation on immunoregulatory cells in the rat mucosal immune response. Clin Exp Immunol. 1985 Apr;60(1):217–224. [PMC free article] [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Lim G. M., Sheldon G. F., Alverdy J. Biliary secretory IgA levels in rats with protein-calorie malnutrition. Ann Surg. 1988 May;207(5):635–640. doi: 10.1097/00000658-198805000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Messiha N., Watson R. R. Immune components of the intestinal mucosae of ageing and protein deficient mice. Immunology. 1981 Jul;43(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., James S. J., Inamizu T., Chang M. P. Immunologic basis for susceptibility to infection in the aged. Gerontology. 1984;30(5):279–289. doi: 10.1159/000212647. [DOI] [PubMed] [Google Scholar]
- McGee D. W., McMurray D. N. The effect of protein malnutrition on the IgA immune response in mice. Immunology. 1988 Jan;63(1):25–29. [PMC free article] [PubMed] [Google Scholar]
- McGee D. W., McMurray D. N. The effect of protein malnutrition on the IgA immune response in mice. Immunology. 1988 Jan;63(1):25–29. [PMC free article] [PubMed] [Google Scholar]
- Neumann C. G., Lawlor G. J., Jr, Stiehm E. R., Swenseid M. E., Newton C., Herbert J., Ammann A. J., Jacob M. Immunologic responses in malnourished children. Am J Clin Nutr. 1975 Feb;28(2):89–104. doi: 10.1093/ajcn/28.2.89. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Sterman D., Ogra P. L. Rotavirus infection in mice: pathogenesis and immunity. Adv Exp Med Biol. 1987;216B:1015–1023. [PubMed] [Google Scholar]
- Roux M. E., del Carmen Lopez M. Impairment of IgA expression and cell mediated immunity observed on Peyer's patches of protein-depleted rats at weaning and then fed on 20% casein. Adv Exp Med Biol. 1987;216A:847–855. doi: 10.1007/978-1-4684-5344-7_99. [DOI] [PubMed] [Google Scholar]
- Scrimshaw N. S., Taylor C. E., Gordon J. E. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- Senda S., Cheng E., Kawanishi H. Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol. 1988 Feb;27(2):157–164. doi: 10.1111/j.1365-3083.1988.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Ontogeny and senescence of salivary immunity. J Dent Res. 1987 Feb;66(2):451–456. doi: 10.1177/00220345870660021101. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Source of IgA in tears of rats. Immunology. 1984 Dec;53(4):791–799. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. The effect of aging on the secretory immune system of the eye. Immunology. 1988 Mar;63(3):403–410. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Colby E. B., Hann L. E., Allansmith M. R., Wira C. R. Production and utilization of a mouse monoclonal antibody to rat IgA: identification of gender-related differences in the secretory immune system. Immunol Invest. 1986 Jun;15(4):311–325. doi: 10.3109/08820138609052950. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Hann L. E. Hormonal influence on the secretory immune system of the eye: endocrine impact on the lacrimal gland accumulation and secretion of IgA and IgG. J Steroid Biochem. 1989;34(1-6):253–262. doi: 10.1016/0022-4731(89)90089-7. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Hann L. E., Yee L., Allansmith M. R. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol (Copenh) 1990 Apr;68(2):188–194. doi: 10.1111/j.1755-3768.1990.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Variations in free secretory component levels in mucosal secretions of the rat. J Immunol. 1983 Mar;130(3):1330–1335. [PubMed] [Google Scholar]
- Sullivan D. A., Yee L., Conner A. S., Hann L. E., Olivier M., Allansmith M. R. Influence of ocular surface antigen on the postnatal accumulation of immunoglobulin-containing cells in the rat lacrimal gland. Immunology. 1990 Dec;71(4):573–580. [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Miura S., Tashiro H., Serizawa H., Hamada Y., Yoshioka M., Tsuchiya M. Morphological alteration of gut-associated lymphoid tissue after long-term total parenteral nutrition in rats. Cell Tissue Res. 1991 Oct;266(1):29–36. doi: 10.1007/BF00678708. [DOI] [PubMed] [Google Scholar]
- Umezawa M., Hanada K., Naiki H., Chen W. H., Hosokawa M., Hosono M., Hosokawa T., Takeda T. Effects of dietary restriction on age-related immune dysfunction in the senescence accelerated mouse (SAM). J Nutr. 1990 Nov;120(11):1393–1400. doi: 10.1093/jn/120.11.1393. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Buts J. P., Lescoat G. Ontogeny of secretory component in rat liver. Immunology. 1989 Nov;68(3):295–299. [PMC free article] [PubMed] [Google Scholar]
- Wade S., Lemonnier D., Alexiu A., Bocquet L. Effect of early postnatal under- and overnutrition on the development of IgA plasma cells in mouse gut. J Nutr. 1982 Jun;112(6):1047–1051. doi: 10.1093/jn/112.6.1047. [DOI] [PubMed] [Google Scholar]
- Watson R. R., McMurray D. N., Martin P., Reyes M. A. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. Am J Clin Nutr. 1985 Aug;42(2):281–288. doi: 10.1093/ajcn/42.2.281. [DOI] [PubMed] [Google Scholar]
- Watson R. R., McMurray D. N. The effects of malnutrition on secretory and cellular immune processes. CRC Crit Rev Food Sci Nutr. 1979 Dec;12(2):113–159. doi: 10.1080/10408397909527275. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sandoe C. P., Steele M. G. Glucocorticoid regulation of the humoral immune system. I. In vivo effects of dexamethasone on IgA and IgG in serum and at mucosal surfaces. J Immunol. 1990 Jan 1;144(1):142–146. [PubMed] [Google Scholar]
- Xie Q. W. Experimental studies on changes of neuroendocrine functions during starvation and refeeding. Neuroendocrinology. 1991;53 (Suppl 1):52–59. doi: 10.1159/000125796. [DOI] [PubMed] [Google Scholar]


