Abstract
The late complement components, apart from their lytic function, are known to trigger the release of various proinflammatory substances from different types of nucleated cells. In the present study, the interaction of C5b-9 with synovial fibroblast cells (SFC) was examined. It was found that incubation of SFC with activated complement components resulted in binding of C5b-9 to the cell membrane; subsequently an increase in abundance of collagenase-specific mRNA was seen, as assessed by Northern blotting. When C8-deficient serum was used as source of complement neither binding of C5b-9 nor an increase in collagenase-specific mRNA could be detected. These findings suggest that C5b-9, which might be generated during rheumatoid inflammation, may contribute to chronic joint destruction by triggering collagenolytic activity.
Full text
PDF

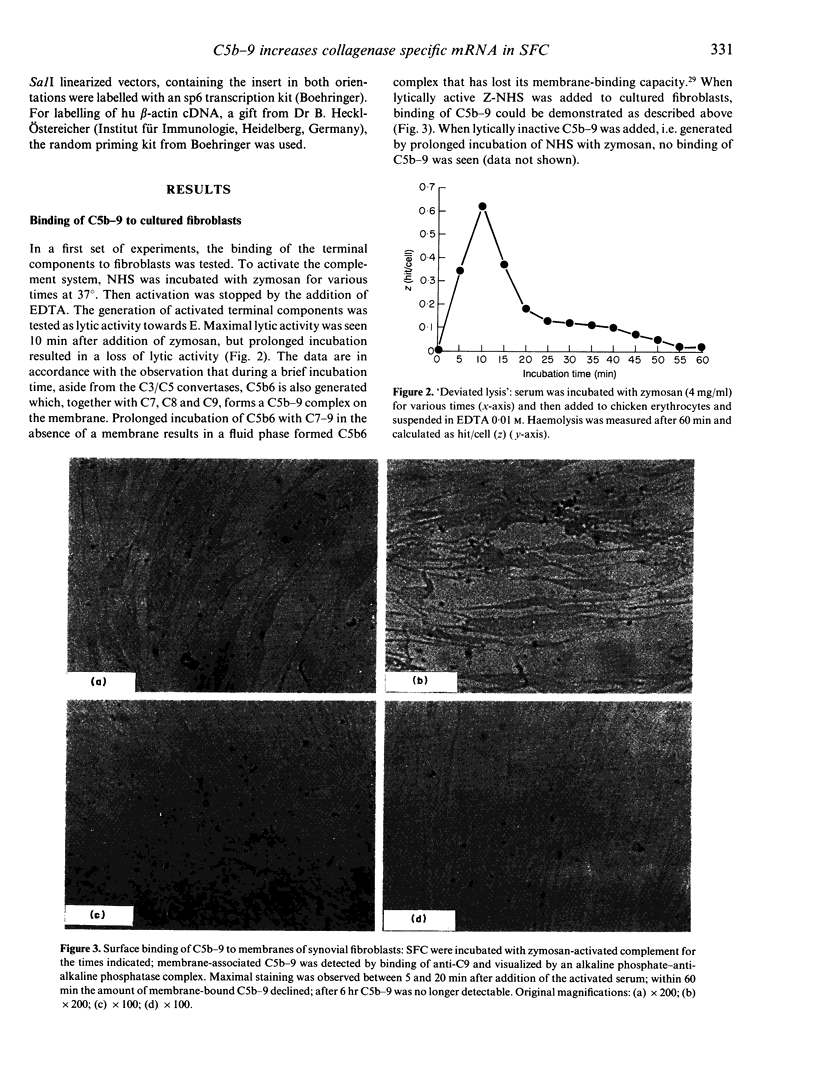
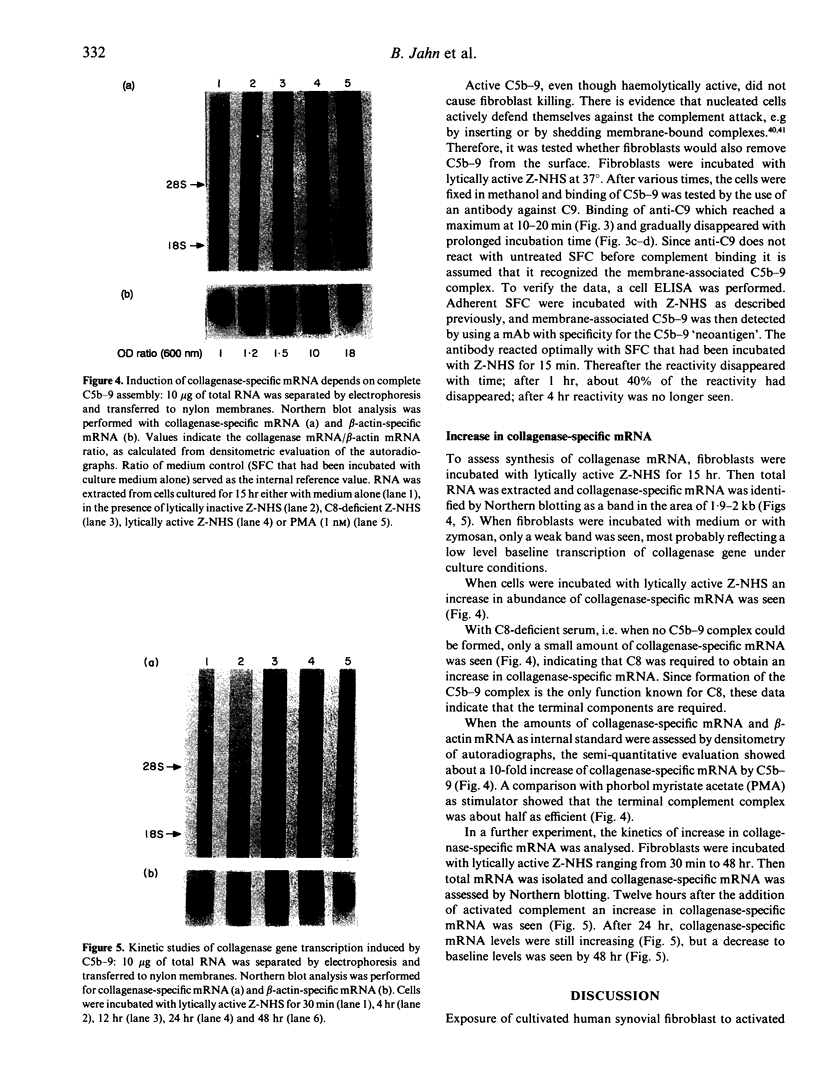


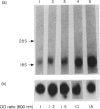
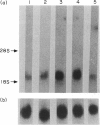
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Mackensen A., Osterholz J., Brugger W., Löhr G. W. Microculture assay for human macrophage maturation in vitro. Cell-ELISA analysis of differentiation antigen expression. Int Arch Allergy Appl Immunol. 1988;86(3):281–287. doi: 10.1159/000234585. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Osofsky S. G. Activation of human complement by heat-killed, human kidney cells grown in cell culture. J Immunol. 1980 Jan;124(1):81–86. [PubMed] [Google Scholar]
- Brinckerhoff C. E., Ruby P. L., Austin S. D., Fini M. E., White H. D. Molecular cloning of human synovial cell collagenase and selection of a single gene from genomic DNA. J Clin Invest. 1987 Feb;79(2):542–546. doi: 10.1172/JCI112845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. F., Koski C. L., Shin M. L. Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol. 1985 Mar;134(3):1804–1809. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol. 1989 May;92(5):699–706. doi: 10.1111/1523-1747.ep12696891. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. Proteinases in connective tissue breakdown. Ciba Found Symp. 1979;(75):87–103. doi: 10.1002/9780470720585.ch6. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Betz M., Günther J., Rother K. O., Sterzel B. The complement membrane attack complex stimulates the prostanoid production of cultured glomerular epithelial cells. Int Arch Allergy Appl Immunol. 1988;85(1):87–93. doi: 10.1159/000234479. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Gemsa D., Resch K. Induction of prostanoid synthesis in human platelets by the late complement components C5b-9 and channel forming antibiotic nystatin: inhibition of the reacylation of liberated arachidonic acid. J Immunol. 1985 Aug;135(2):1320–1324. [PubMed] [Google Scholar]
- Hänsch G. M., Seitz M., Betz M. Effect of the late complement components C5b-9 on human monocytes: release of prostanoids, oxygen radicals and of a factor inducing cell proliferation. Int Arch Allergy Appl Immunol. 1987;82(3-4):317–320. doi: 10.1159/000234216. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Seitz M., Martinotti G., Betz M., Rauterberg E. W., Gemsa D. Macrophages release arachidonic acid, prostaglandin E2, and thromboxane in response to late complement components. J Immunol. 1984 Oct;133(4):2145–2150. [PubMed] [Google Scholar]
- Hänsch G. M. The complement attack phase: control of lysis and non-lethal effects of C5b-9. Immunopharmacology. 1992 Sep-Oct;24(2):107–117. doi: 10.1016/0162-3109(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Keller H., Löke S., Hänsch G. M., Jentschura D., Gerhard H., Heene D. L. Rezidivierende Meningitis bei familiärem Mangel der achten Komponente des Komplementsystems. Klin Wochenschr. 1987 Apr 15;65(8):387–390. doi: 10.1007/BF01745581. [DOI] [PubMed] [Google Scholar]
- Koski C. L., Ramm L. E., Hammer C. H., Mayer M. M., Shin M. L. Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3816–3820. doi: 10.1073/pnas.80.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Haensch G. M., Goppelt M., Resch K., Gemsa D. Activation of glomerular mesangial cells by the terminal membrane attack complex of complement. J Immunol. 1987 Apr 15;138(8):2473–2480. [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Frøland S. S., Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985 Aug;22(2):197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Frøland S. S., Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985 Aug;22(2):197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Paus A. Complement activation in synovial fluid and tissue from patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1986 Nov;29(11):1359–1364. doi: 10.1002/art.1780291108. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes P. A., Maluf J. G., Curd J. G., Vaughan J. H. Association of rheumatoid factor with complement activation in rheumatoid arthritis and other diseases. Clin Exp Immunol. 1983 Aug;53(2):391–396. [PMC free article] [PubMed] [Google Scholar]
- Rother U., Hänsch G., Menzel J., Rother K. Deviated lysis: transfer of complement lytic activity to unsensitized cells. I. Generation of the transferable activity on the surface of complement resistant bacteria. Z Immunitatsforsch Exp Klin Immunol. 1974 Nov;148(2):172–186. [PubMed] [Google Scholar]
- Sandy J. D., Brown H. L., Lowther D. A. Degradation of proteoglycan in articular cartilage. Biochim Biophys Acta. 1978 Nov 1;543(4):536–544. doi: 10.1016/0304-4165(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Seeger W., Suttorp N., Hellwig A., Bhakdi S. Noncytolytic terminal complement complexes may serve as calcium gates to elicit leukotriene B4 generation in human polymorphonuclear leukocytes. J Immunol. 1986 Aug 15;137(4):1286–1293. [PubMed] [Google Scholar]
- Steckel E. W., York R. G., Monahan J. B., Sodetz J. M. The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem. 1980 Dec 25;255(24):11997–12005. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbohm I., Schönermark M., Wingen A. M., Berger B., Rother K., Hänsch G. M. C5b-8 and C5b-9 modulate the collagen release of human glomerular epithelial cells. Kidney Int. 1990 Apr;37(4):1098–1104. doi: 10.1038/ki.1990.91. [DOI] [PubMed] [Google Scholar]
- Truppe W., Kresse H. Uptake of proteoglycans and sulfated glycosaminoglycans by cultured skin fibroblasts. Eur J Biochem. 1978 Apr 17;85(2):351–356. doi: 10.1111/j.1432-1033.1978.tb12246.x. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Williams J. D., Czop J. K., Abrahamson D. R., Davies M., Austen K. F. Activation of the alternative complement pathway by isolated human glomerular basement membrane. J Immunol. 1984 Jul;133(1):394–399. [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]
- von Kempis J., Torbohm I., Schönermark M., Jahn B., Seitz M., Hänsch G. M. Effect of the late complement components C5b-9 and of platelet-derived growth factor on the prostaglandin release of human synovial fibroblast-like cells. Int Arch Allergy Appl Immunol. 1989;90(3):248–255. doi: 10.1159/000235032. [DOI] [PubMed] [Google Scholar]