Abstract
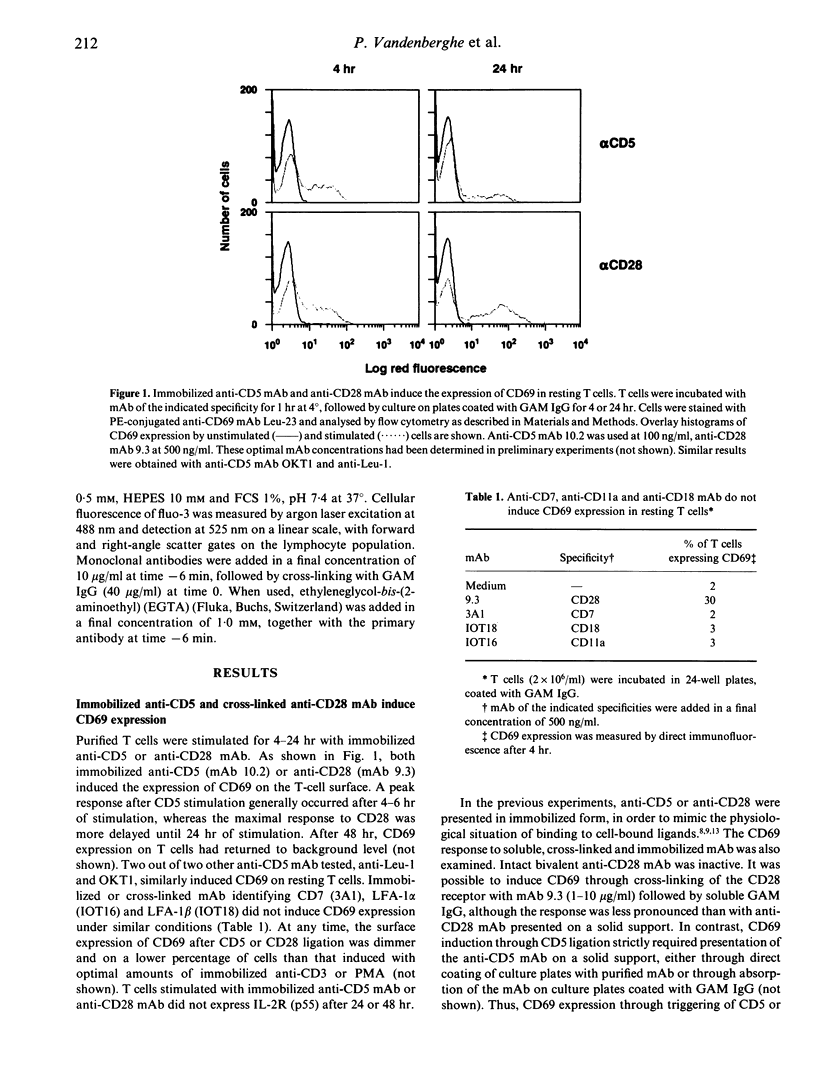
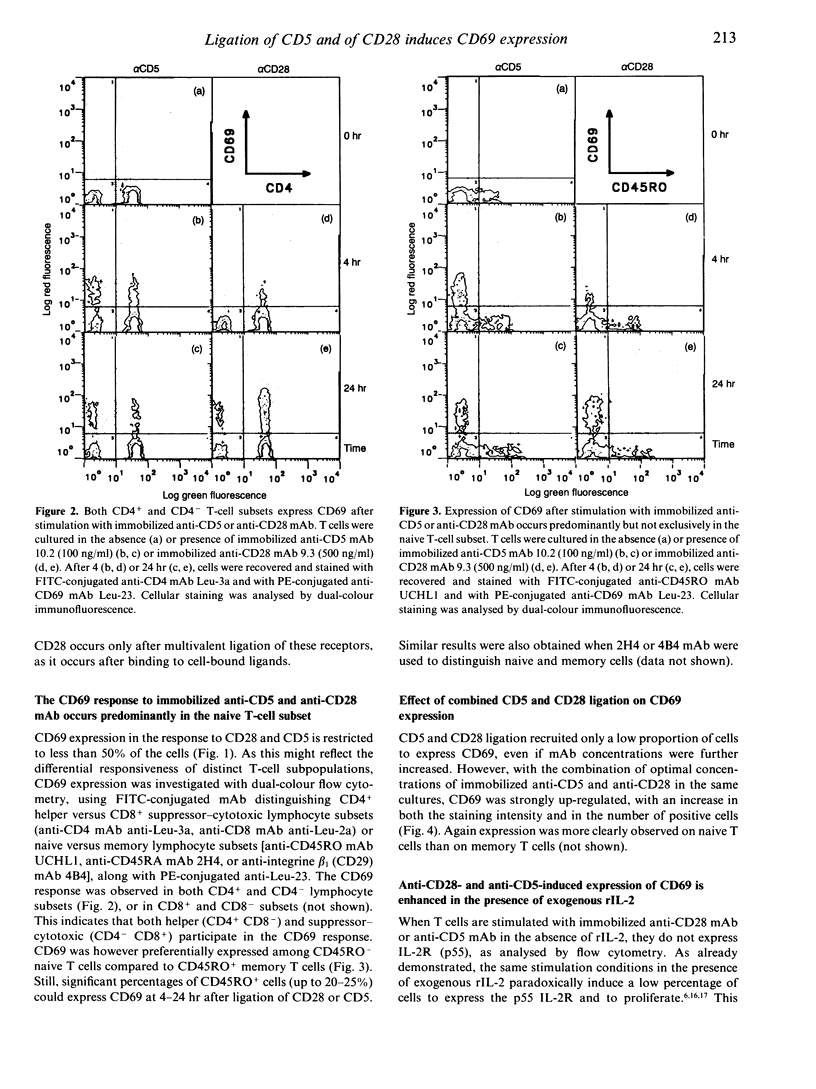
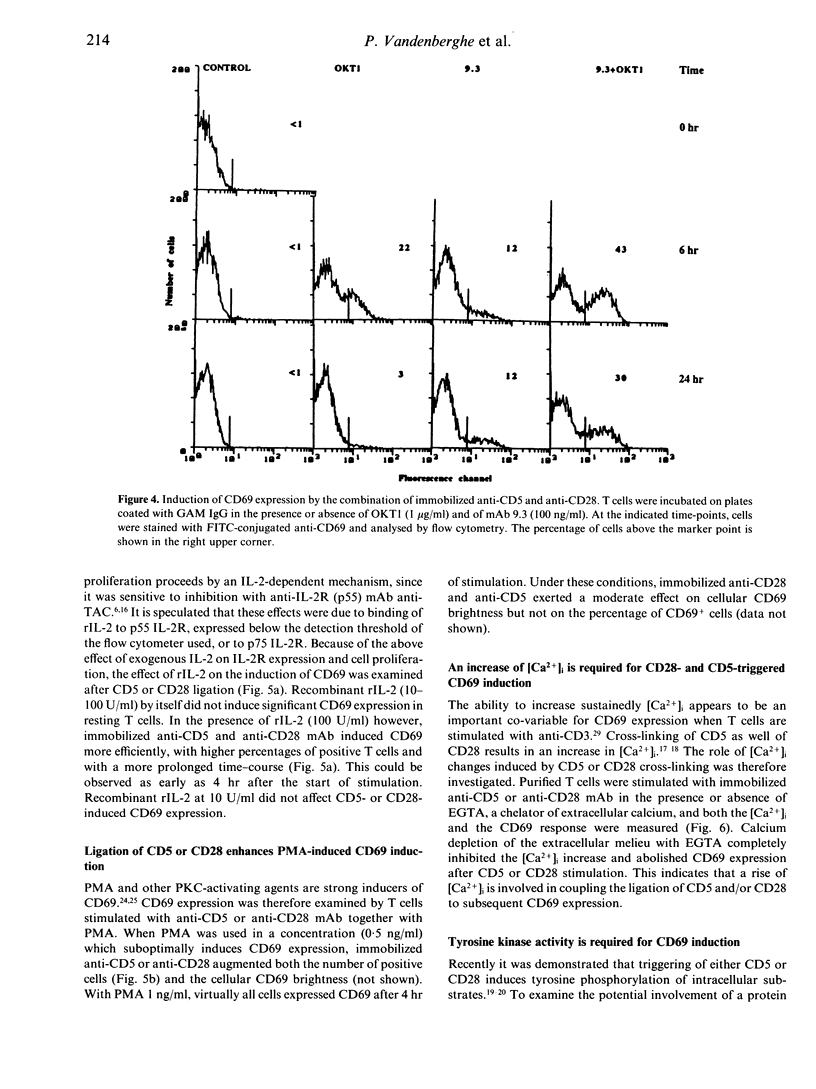
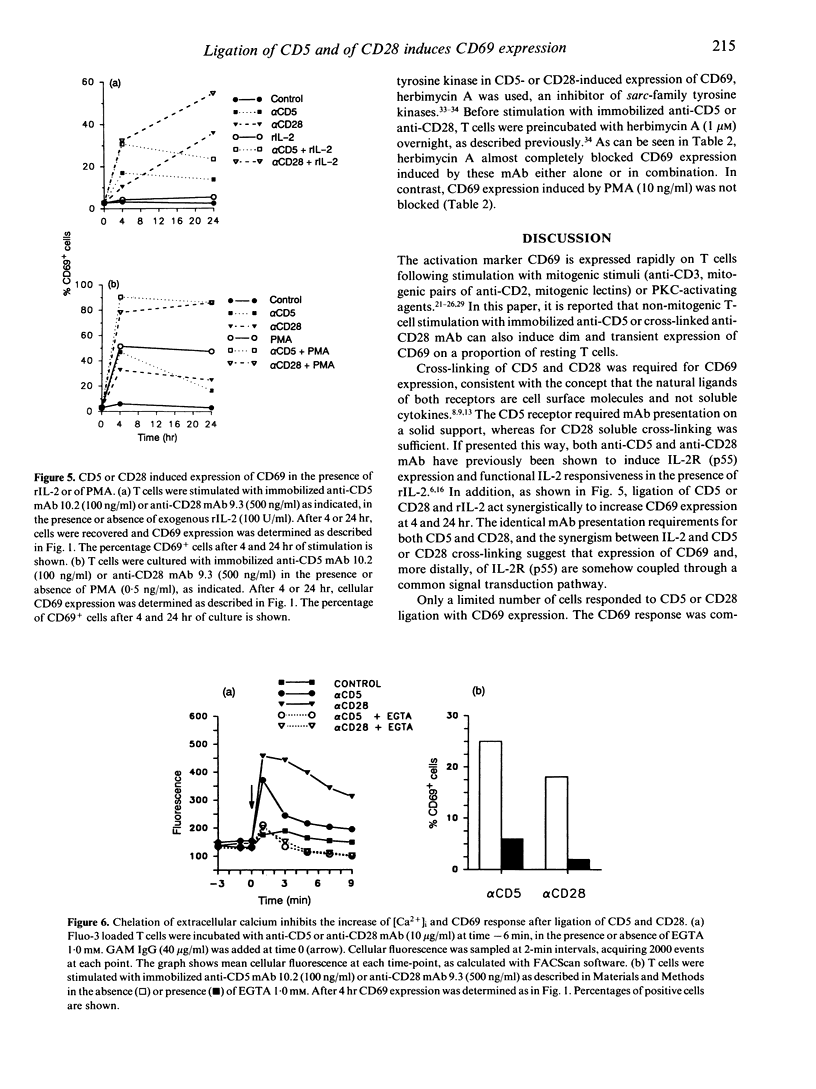
The CD5 and CD28 molecules on T lymphocytes can each exert an accessory role in T-cell activation. Ligands for CD5 and CD28 have been identified as CD72 and B7/BB1 respectively. The function of, and the signal transduction pathways coupled to CD28 have been the subject of extensive studies. In contrast, it is still debated whether CD5 functions as a receptor which directly transduces an independent signal to the T cell. In this paper, it is reported that culture of purified T cells in the presence of either immobilized anti-CD5 monoclonal antibody (mAb) (OKT1, Leu-1 or 10.2) or cross-linked anti-CD28 (9.3) mAb (but not of anti-LFA-1 alpha, anti-LFA-1 beta, or anti-CD7) induces expression of CD69, an early activation marker, in the absence of other activating stimuli. CD69 expression was consistently detectable after 3-24 hr on 20-50% of T cells, within both the CD4 and CD8 subsets. CD45RO- CD45RA+ naive T cells were more responsive than CD45RO+ CD45RA- memory T cells. In the presence of recombinant (r) interleukin-2 (IL-2), anti-CD5- or anti-CD28- induced CD69 expression was further up-regulated, more sustained and, as previously shown, succeeded by IL-2 responsiveness. Simultaneous cross-linking of both CD5 and CD28 enhanced CD69 expression above the levels obtained with optimal amounts of both ligands separately. In the presence of a submitogenic dose of the protein kinase C (PKC) activating agent phorbol 12-myristate 13-acetate (PMA), co-stimulation with anti-CD5 or anti-CD28 increased CD69 expression above that induced by PMA alone. Cross-linking of CD5 or CD28 induces an early rise of cytoplasmic free calcium concentration ([Ca2+)]i) and both this rise and CD69 expression were inhibited by chelation of extracellular Ca2+ with ethyleneglycol-bis-(2-aminoethyl)-tetraacetate (EGTA). Pretreatment of the cells with the tyrosine kinase inhibitor herbimycin A also blocked CD69 expression. The data thus antigen-independent fashion. Moreover it is demonstrated that influx of Ca2+ and tyrosine kinase activity are involved in the signal transduction pathways of both receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberola-Ila J., Places L., Cantrell D. A., Vives J., Lozano F. Intracellular events involved in CD5-induced human T cell activation and proliferation. J Immunol. 1992 Mar 1;148(5):1287–1293. [PubMed] [Google Scholar]
- Baroja M. L., Ceuppens J. L., Van Damme J., Billiau A. Cooperation between an anti-T cell (anti-CD28) monoclonal antibody and monocyte-produced IL-6 in the induction of T cell responsiveness to IL-2. J Immunol. 1988 Sep 1;141(5):1502–1507. [PubMed] [Google Scholar]
- Baroja M. L., Lorre K., Van Vaeck F., Ceuppens J. L. The anti-T cell monoclonal antibody 9.3 (anti-CD28) provides a helper signal and bypasses the need for accessory cells in T cell activation with immobilized anti-CD3 and mitogens. Cell Immunol. 1989 Apr 15;120(1):205–217. doi: 10.1016/0008-8749(89)90188-3. [DOI] [PubMed] [Google Scholar]
- Bjorndahl J. M., Nakamura S., Hara T., Jung L. K., Fu S. M. The 28-kDa/32-kDa activation antigen EA 1. Further characterization and signal requirements for its expression. J Immunol. 1988 Dec 15;141(12):4094–4100. [PubMed] [Google Scholar]
- Cebrián M., Redondo J. M., López-Rivas A., Rodríguez-Tarduchy G., De Landázuri M. O., Sánchez-Madrid F. Expression and function of AIM, an activation inducer molecule of human lymphocytes, is dependent on the activation of protein kinase C. Eur J Immunol. 1989 May;19(5):809–815. doi: 10.1002/eji.1830190505. [DOI] [PubMed] [Google Scholar]
- Cebrián M., Yagüe E., Rincón M., López-Botet M., de Landázuri M. O., Sánchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988 Nov 1;168(5):1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Cosulich M. E., Rubartelli A., Risso A., Cozzolino F., Bargellesi A. Functional characterization of an antigen involved in an early step of T-cell activation. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Activation of T lymphocytes by immobilized monoclonal antibodies to CD3. Regulatory influences of monoclonal antibodies to additional T cell surface determinants. J Clin Invest. 1988 May;81(5):1497–1505. doi: 10.1172/JCI113481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Jung L. K., Bjorndahl J. M., Fu S. M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986 Dec 1;164(6):1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- June C. H., Rabinovitch P. S., Ledbetter J. A. CD5 antibodies increase intracellular ionized calcium concentration in T cells. J Immunol. 1987 May 1;138(9):2782–2792. [PubMed] [Google Scholar]
- Jung L. K., Haynes B. F., Nakamura S., Pahwa S., Fu S. M. Expression of early activation antigen (CD69) during human thymic development. Clin Exp Immunol. 1990 Sep;81(3):466–474. doi: 10.1111/j.1365-2249.1990.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Buck D. W., Rhodes L., Ding A., Evans E., Barney C., Phillips J. H. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988 May 1;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Imboden J. B., Schieven G. L., Grosmaire L. S., Rabinovitch P. S., Lindsten T., Thompson C. B., June C. H. CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood. 1990 Apr 1;75(7):1531–1539. [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Martin P. J., Spooner C. E., Wofsy D., Tsu T. T., Beatty P. G., Gladstone P. Antibodies to Tp67 and Tp44 augment and sustain proliferative responses of activated T cells. J Immunol. 1985 Oct;135(4):2331–2336. [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzer S. J., Guyre P. M., Burakoff S. J., Faller D. V. Spontaneous aggregation as a mechanism for human monocyte purification. Cell Immunol. 1986 Sep;101(2):312–319. doi: 10.1016/0008-8749(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Risso A., Cosulich M. E., Rubartelli A., Mazza M. R., Bargellesi A. MLR3 molecule is an activation antigen shared by human B, T lymphocytes and T cell precursors. Eur J Immunol. 1989 Feb;19(2):323–328. doi: 10.1002/eji.1830190216. [DOI] [PubMed] [Google Scholar]
- Risso A., Smilovich D., Capra M. C., Baldissarro I., Yan G., Bargellesi A., Cosulich M. E. CD69 in resting and activated T lymphocytes. Its association with a GTP binding protein and biochemical requirements for its expression. J Immunol. 1991 Jun 15;146(12):4105–4114. [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Testi R., Phillips J. H., Lanier L. L. Constitutive expression of a phosphorylated activation antigen (Leu 23) by CD3bright human thymocytes. J Immunol. 1988 Oct 15;141(8):2557–2563. [PubMed] [Google Scholar]
- Testi R., Phillips J. H., Lanier L. L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989 Mar 15;142(6):1854–1860. [PubMed] [Google Scholar]
- Turka L. A., Ledbetter J. A., Lee K., June C. H., Thompson C. B. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990 Mar 1;144(5):1646–1653. [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Vandenberghe P. A., Ceuppens J. L. Flow cytometric measurement of cytoplasmic free calcium in human peripheral blood T lymphocytes with fluo-3, a new fluorescent calcium indicator. J Immunol Methods. 1990 Mar 9;127(2):197–205. doi: 10.1016/0022-1759(90)90069-8. [DOI] [PubMed] [Google Scholar]
- Vandenberghe P., Ceuppens J. L. Immobilized anti-CD5 together with prolonged activation of protein kinase C induce interleukin 2-dependent T cell growth: evidence for signal transduction through CD5. Eur J Immunol. 1991 Feb;21(2):251–259. doi: 10.1002/eji.1830210203. [DOI] [PubMed] [Google Scholar]
- Vandenberghe P., Freeman G. J., Nadler L. M., Fletcher M. C., Kamoun M., Turka L. A., Ledbetter J. A., Thompson C. B., June C. H. Antibody and B7/BB1-mediated ligation of the CD28 receptor induces tyrosine phosphorylation in human T cells. J Exp Med. 1992 Apr 1;175(4):951–960. doi: 10.1084/jem.175.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwilghen J., Vandesande R., Vandenberghe P., Ceuppens J. L. Crosslinking of the CD5 antigen on human T cells induces functional IL2 receptors. Cell Immunol. 1990 Nov;131(1):109–119. doi: 10.1016/0008-8749(90)90238-m. [DOI] [PubMed] [Google Scholar]


