Abstract
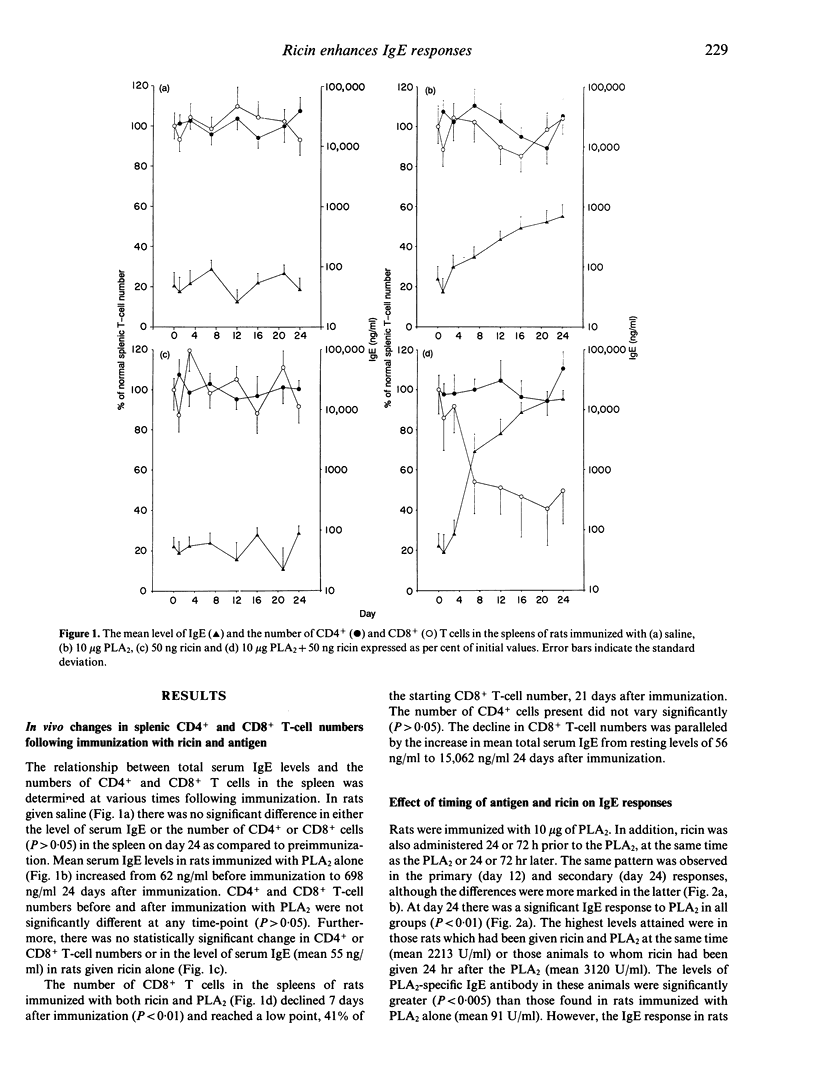
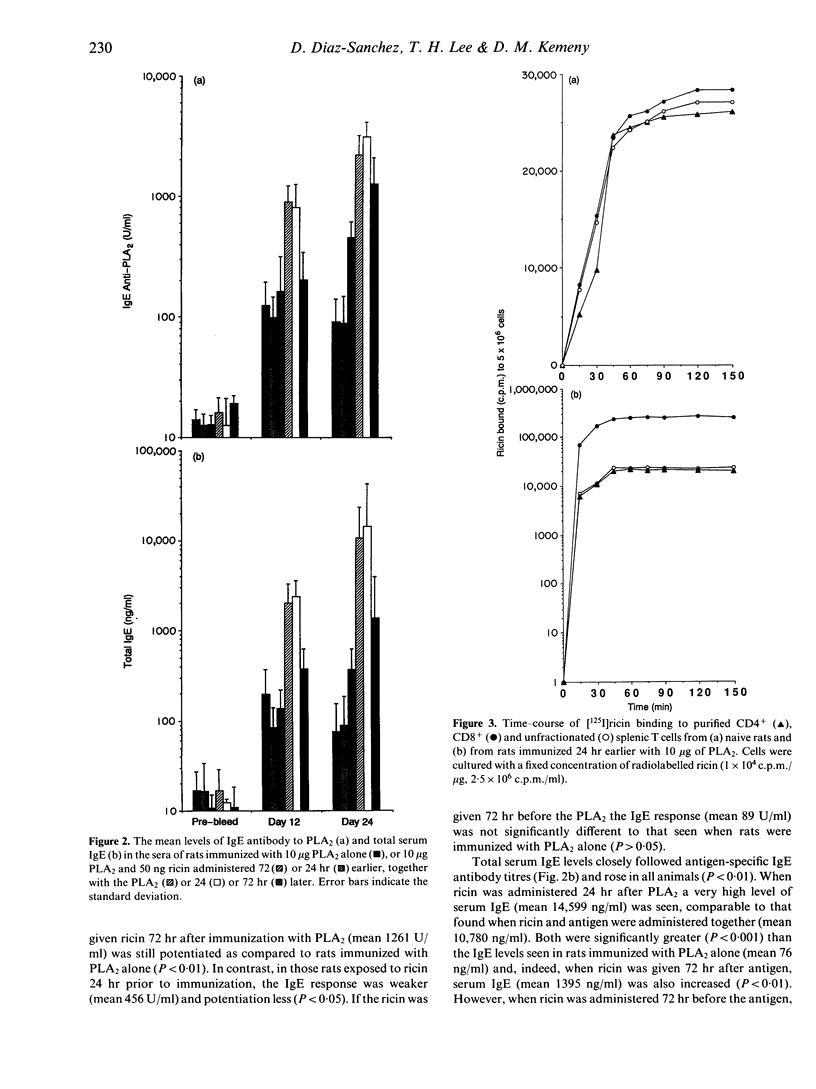
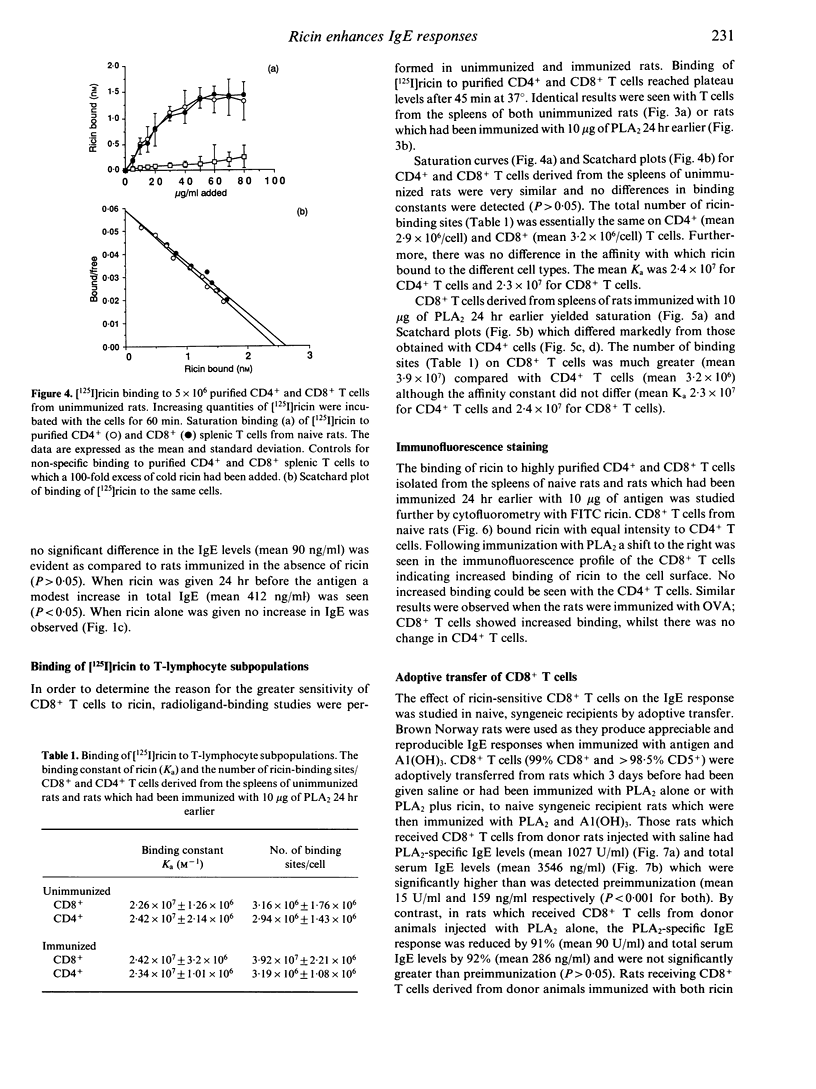
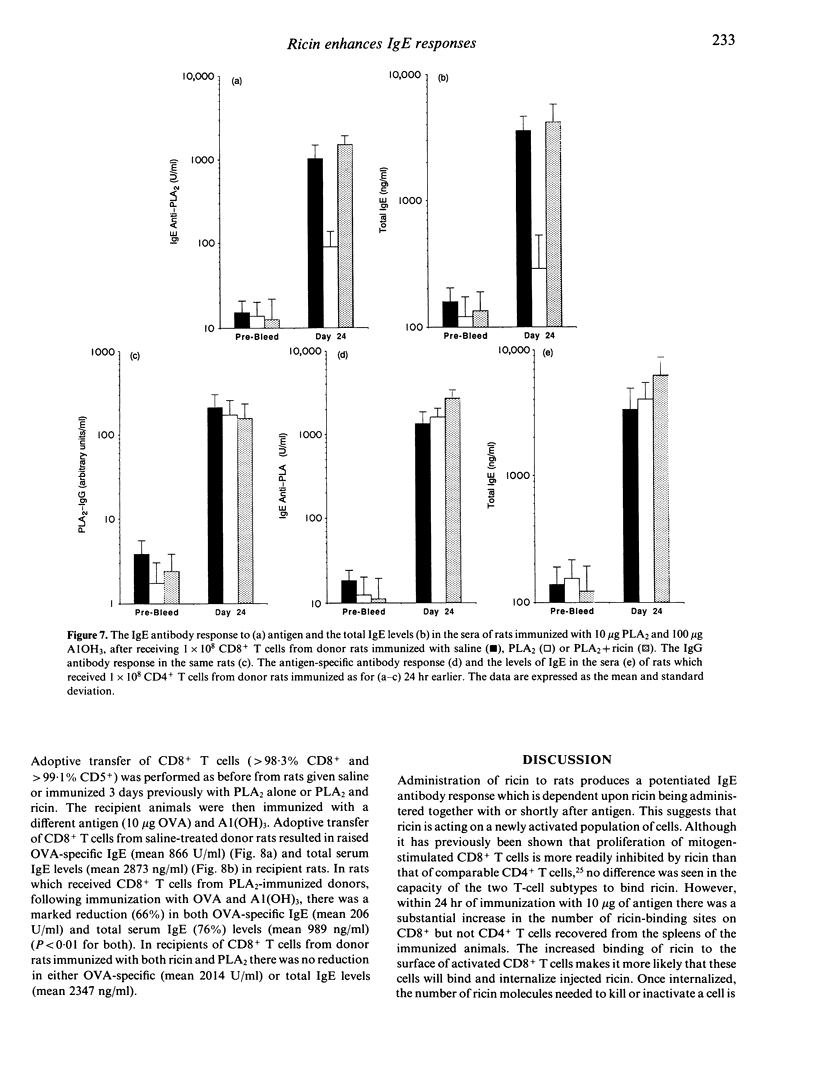
Ricin, a toxic lectin from castor beans greatly enhances IgE responses to bee venom phospholipase A2 (PLA2) in high and low IgE responder strains of rat. The increase in IgE is accompanied by a 60% reduction in the number of CD8+ but not CD4+ T cells in the spleen. Optimal enhancement of IgE by ricin occurs when it is given at the same time as the antigen or 24 hr later, suggesting that it acts on cells which were activated as a consequence of immunization. Radio ligand-binding studies with 125I ricin were used to compare the number of ricin binding sites on CD4+ and CD8+ T cells. No difference was seen in either the affinity or the number of receptors for ricin on the CD4+ and CD8+ T cells of unimmunized rats. In contrast, CD8+ T cells taken from rats which had been immunized with 10 micrograms of PLA2 24 hr earlier demonstrated considerably more ricin receptors (3.9 x 10(7) +/- 2.2 x 10(6) binding sites/cell) than CD4+ T cells (3.19 x 10(6) +/- 1.08 x 10(6) binding sites/cell). However the affinity of the receptors for ricin was unchanged. Cytofluorographic analysis with fluorescein isothiocyanate (FITC)-labelled ricin confirmed these observations and indicated that increased ricin binding occurred on a subpopulation of CD8+ T cells. The effect of CD8+ T cells on IgE regulation was investigated by adoptive transfer. 1 x 10(8) highly purified (> 98%) splenic CD8+ T cells collected from Brown Norway rats 3 days after immunization with 10 micrograms of PLA2 were adoptively transferred to naive, syngeneic recipients. The IgE antibody response to PLA2 + A1(OH)3 seen in these animals was reduced by 91%. Adoptive transfer of CD4+ T cells from the same donor animals did not induce suppression and nor did adoptive transfer of CD8+ T cells from animals given both ricin and PLA2. However, when recipients of CD8+ T cells taken from rats immunized with PLA2 were immunized with a different antigen [ovalbumin (OVA)] and A1(OH)3 the IgE antibody response was also suppressed, although to a lesser extent (66%). These results show that co-administration of ricin and PLA2 depletes a subpopulation of ricin-sensitive, early activated CD8+ T cells and that these CD8+ T cells are potent suppressors of the primary IgE response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Winn H. J. Murine CD8+ T cell helper function is particularly sensitive to cyclosporine suppression in vivo. J Immunol. 1989 Dec 15;143(12):3940–3943. [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Stanescu G., Magalski A. E., Qian Y. Y. Cyclosporin A is an adjuvant in murine IgE antibody responses. J Immunol. 1989 Jun 15;142(12):4225–4232. [PubMed] [Google Scholar]
- Cheng I. K., Dorsch S. E., Hall B. M. The regulation of autoantibody production in Heymann's nephritis by T lymphocyte subsets. Lab Invest. 1988 Dec;59(6):780–788. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. Generation of a long-lived IgE response in high and low responder strains of rat by co-administration of ricin and antigen. Immunology. 1991 Feb;72(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. The sensitivity of rat CD8+ and CD4+ T cells to ricin in vivo and in vitro and their relationship to IgE regulation. Immunology. 1990 Jan;69(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Ellerman K. E., Powers J. M., Brostoff S. W. A suppressor T-lymphocyte cell line for autoimmune encephalomyelitis. Nature. 1988 Jan 21;331(6153):265–267. doi: 10.1038/331265a0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Fowlkes B. J., Waxdal M. J., Sharrow S. O., Thomas C. A., 3rd, Asofsky R., Mathieson B. J. Differential binding of fluorescein-labeled lectins to mouse thymocytes: subsets revealed by flow microfluorometry. J Immunol. 1980 Aug;125(2):623–630. [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973 Sep 1;138(3):538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Batty J. E., Turner K. J. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 1981 Mar;42(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Leivers S., Warner L. A. Optimal culture conditions for in vitro antigen-induced proliferation of rat lymph node cells. J Immunol Methods. 1981;44(2):205–209. doi: 10.1016/0022-1759(81)90348-3. [DOI] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Finkelman F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988 May 1;140(9):3206–3211. [PubMed] [Google Scholar]
- Kemeny D. M., Frankland A. W., Fahkri Z. I., Trull A. K. Allergy to castor bean in the Sudan: measurement of serum IgE and specific IgE antibodies. Clin Allergy. 1981 Sep;11(5):463–471. doi: 10.1111/j.1365-2222.1981.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Richards D. Increased speed and sensitivity, and reduced sample size of a micro-radioallergosorbent test (MRAST). J Immunol Methods. 1988 Apr 6;108(1-2):105–113. doi: 10.1016/0022-1759(88)90408-5. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Urbanek R., Samuel D., Richards D. Improved sensitivity and specificity of sandwich, competitive and capture enzyme-linked immunosorbent assays for allergen-specific antibodies. Int Arch Allergy Appl Immunol. 1985;77(1-2):198–200. doi: 10.1159/000233785. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Schon-Hegrad M., Oliver J., Girn B., Holt P. G. Regulation of IgE responses to inhaled antigens: cellular mechanisms underlying allergic sensitization versus tolerance induction. Int Arch Allergy Appl Immunol. 1991;94(1-4):78–82. doi: 10.1159/000235331. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- ORDMAN D. An outbreak of bronchial asthma in South Africa, affecting more than 200 persons, caused by castor bean dust from an oil-processing factory. Int Arch Allergy Appl Immunol. 1955;7(1):10–24. doi: 10.1159/000228201. [DOI] [PubMed] [Google Scholar]
- Okudaira H., Sakurai Y., Terada K., Terada E., Ogita T., Miyamoto T. Cyclosporin A-induced suppression of ongoing IgE antibody formation in the mouse. Int Arch Allergy Appl Immunol. 1986;79(2):164–168. doi: 10.1159/000233965. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T., Ochiai T. Effect of antithymocyte serum on reaginic antibody formation in the rat. Immunology. 1974 Feb;26(2):257–268. [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. 3. Effect of thymectomy and splenectomy. J Immunol. 1971 Apr;106(4):1019–1025. [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Pelletier L., Rossert J., Pasquier R., Vial M. C., Druet P. Role of CD8+ T cells in mercury-induced autoimmunity or immunosuppression in the rat. Scand J Immunol. 1990 Jan;31(1):65–74. doi: 10.1111/j.1365-3083.1990.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Prouvost-Danon A., Abadie A., Sapin C., Bazin H., Druet P. Induction of IgE synthesis and potentiation of anti-ovalbumin IgE antibody response by HgCl2 in the rat. J Immunol. 1981 Feb;126(2):699–792. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976 Jul 10;251(13):3977–3984. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Induction of IgE-secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985 Aug;94(1):182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Kinetics and distribution of antigen-specific IgE-secreting cells during the primary antibody response in the rat. J Exp Med. 1983 Jun 1;157(6):2178–2183. doi: 10.1084/jem.157.6.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons B. M., Stahl P. D., Russell J. H. Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem. 1986 Jun 15;261(17):7912–7920. [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988 Jan 1;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Ben-Nun A., Wekerle H. Regulatory circuits in autoimmunity: recruitment of counter-regulatory CD8+ T cells by encephalitogenic CD4+ T line cells. Eur J Immunol. 1988 Dec;18(12):1993–1999. doi: 10.1002/eji.1830181219. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Regulation of homocytotropic antibody formation in the rat. IV. Effects of various immunosuppressive drugs. J Immunol. 1971 Aug;107(2):579–585. [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R. C., McGurl B., Lord M. Allergy to castor bean. II. Identification of the major allergens in castor bean seeds. J Allergy Clin Immunol. 1988 Jul;82(1):67–72. doi: 10.1016/0091-6749(88)90053-x. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R., Lessof M. H. Allergy to castor bean. I. Its relationship to sensitization to common inhalant allergens (atopy). J Allergy Clin Immunol. 1988 Jul;82(1):62–66. doi: 10.1016/0091-6749(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R., Lessof M. H. The relationship between total serum IgE and castor-bean-specific IgE antibodies in castor-bean-sensitive patients from Marseilles. Int Arch Allergy Appl Immunol. 1987;82(3-4):456–460. doi: 10.1159/000234253. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Murdoch R. D., Kemeny D. M. The effect of the castor bean toxin, ricin, on rat IgE and IgG responses. Immunology. 1989 Nov;68(3):307–311. [PMC free article] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]


