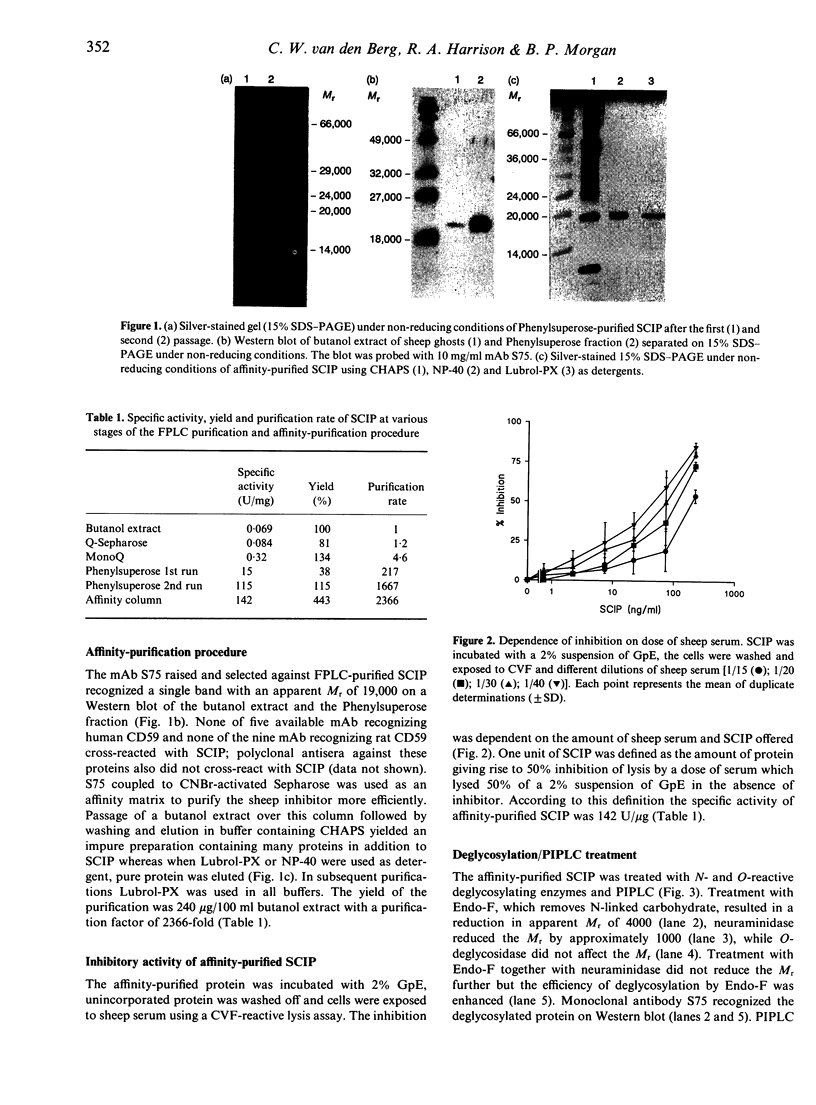
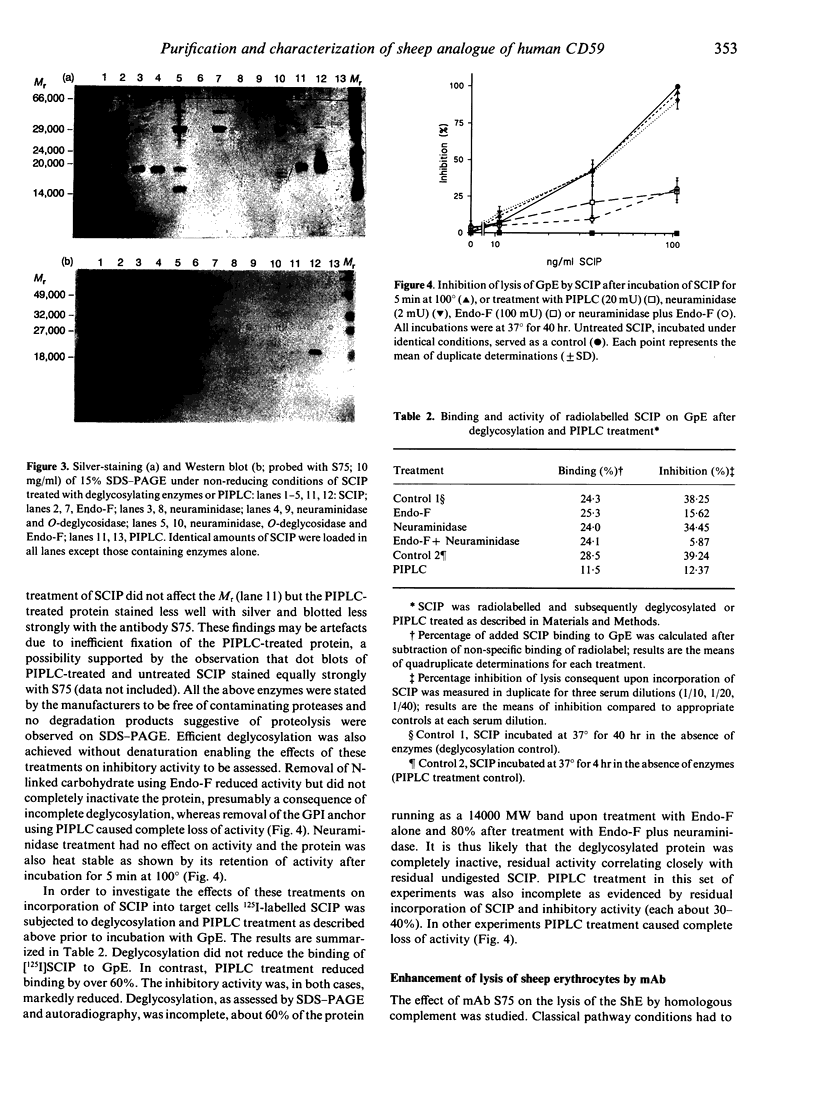
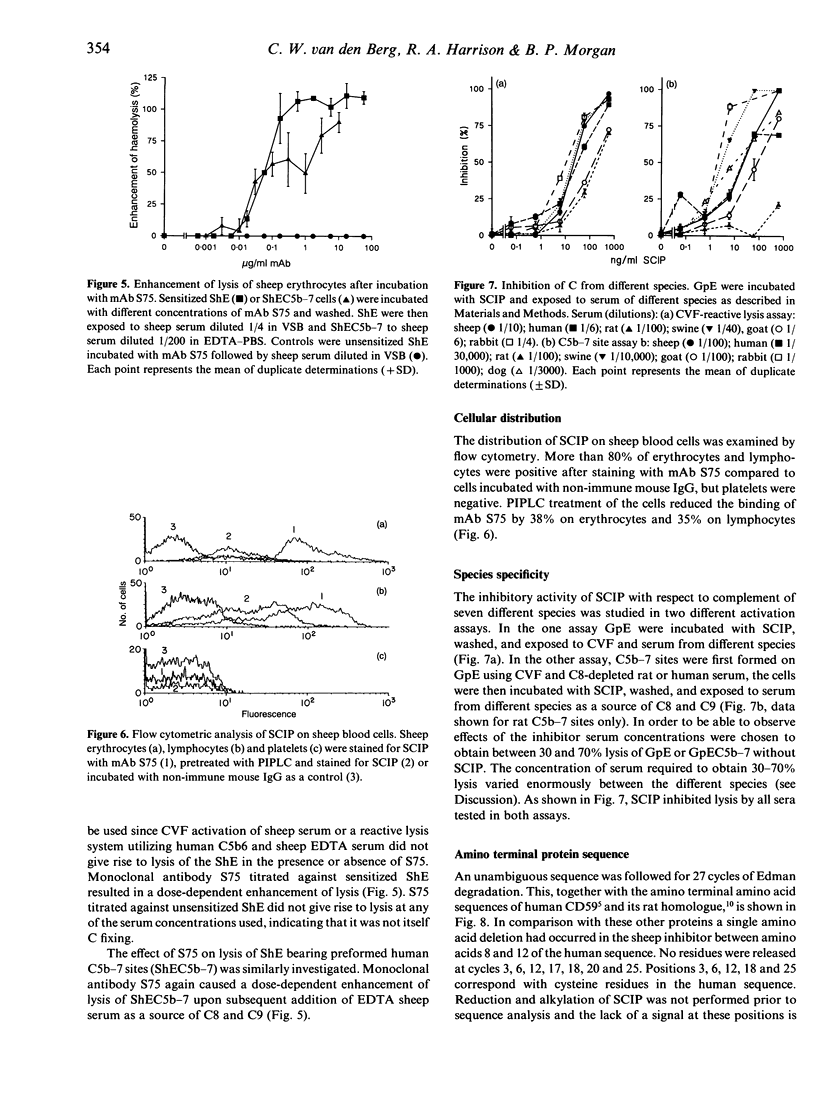
Abstract
An inhibitor of the membrane attack complex of complement was isolated from the membranes of sheep erythrocytes. Fast protein liquid chromatography (FPLC) and affinity purification procedures for this sheep complement-inhibiting protein (SCIP) both yielded a pure protein with an apparent M(r) of 19,000 under reducing and non-reducing conditions. Incubation of the denatured protein with neuraminidase and Endo-F reduced the apparent M(r) to 18,000 and 15,000 respectively, while treatment with O-deglycosidase or phosphatidylinositol-specific phospholipase C (PIPLC) did not affect the apparent M(r). SCIP was detectable on erythrocytes and lymphocytes but not on platelets and could partially be removed by PIPLC treatment. Deglycosylation of the pure protein markedly reduced and PIPLC treatment abolished its activity. A monoclonal antibody (mAb) raised against sheep complement-inhibiting protein (SCIP) enhanced the susceptibility of sheep erythrocytes to lysis by homologous complement. SCIP inhibited complement after the stage of C5b-7 formation. Amino-terminal protein sequence was obtained and was shown to be similar to that of human CD59. All these features suggest that SCIP is the sheep equivalent of human CD59. Human CD59 has been reported to be species selective in that it inhibits complement from relatively few species. However, SCIP efficiently inhibited lysis of guinea-pig erythrocytes by complement from a wide range of species tested indicating that it is a potent and non-selective inhibitor of the membrane attack complex of complement (MAC).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalmasso A. P., Platt J. L., Bach F. H. Reaction of complement with endothelial cells in a model of xenotransplantation. Clin Exp Immunol. 1991 Oct;86 (Suppl 1):31–35. [PMC free article] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Harada R., Okada N., Fujita T., Okada H. Purification of 1F5 antigen that prevents complement attack on homologous cell membranes. J Immunol. 1990 Mar 1;144(5):1823–1828. [PubMed] [Google Scholar]
- Hein W. R., Beya M. F. Evidence that the B5 molecule on sheep lymphocytes is a homologue of mouse Ly 6 and human CD59. Immunology. 1989 Dec;68(4):580–582. [PMC free article] [PubMed] [Google Scholar]
- Hein W. R., Mackay C. R. Other surface antigens identified on sheep leukocytes. Vet Immunol Immunopathol. 1991 Jan;27(1-3):115–118. doi: 10.1016/0165-2427(91)90090-y. [DOI] [PubMed] [Google Scholar]
- Hein W. R., McClure S., Beya M. F., Dudler L., Trnka Z. A novel glycoprotein expressed on sheep T and B lymphocytes is involved in a T cell activation pathway. J Immunol. 1988 May 1;140(9):2869–2875. [PubMed] [Google Scholar]
- Holguin M. H., Wilcox L. A., Bernshaw N. J., Rosse W. F., Parker C. J. Erythrocyte membrane inhibitor of reactive lysis: effects of phosphatidylinositol-specific phospholipase C on the isolated and cell-associated protein. Blood. 1990 Jan 1;75(1):284–289. [PubMed] [Google Scholar]
- Hughes T. R., Piddlesden S. J., Williams J. D., Harrison R. A., Morgan B. P. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992 May 15;284(Pt 1):169–176. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J. The control of homologous lysis. Immunol Today. 1991 Sep;12(9):312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P. Isolation and characterization of the complement-inhibiting protein CD59 antigen from platelet membranes. Biochem J. 1992 Mar 1;282(Pt 2):409–413. doi: 10.1042/bj2820409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Okada H., Nagami Y., Takahashi K., Okada N., Hideshima T., Takizawa H., Kondo J. 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1553–1559. doi: 10.1016/0006-291x(89)90852-8. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Sims P. J., Rollins S. A., Wiedmer T. Regulatory control of complement on blood platelets. Modulation of platelet procoagulant responses by a membrane inhibitor of the C5b-9 complex. J Biol Chem. 1989 Nov 15;264(32):19228–19235. [PubMed] [Google Scholar]
- Stefanová I., Horejsí V. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J Immunol. 1991 Sep 1;147(5):1587–1592. [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Funahashi Y., Ikezawa H., Nakashima I. Analysis of PI (phosphatidylinositol)-anchoring antigens in a patient of paroxysmal nocturnal hemoglobinuria (PNH) reveals deficiency of 1F5 antigen (CD59), a new complement-regulatory factor. FEBS Lett. 1990 Feb 12;261(1):142–146. doi: 10.1016/0014-5793(90)80656-4. [DOI] [PubMed] [Google Scholar]
- Vogel C. W., Müller-Eberhard H. J. Cobra venom factor: improved method for purification and biochemical characterization. J Immunol Methods. 1984 Oct 12;73(1):203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- Whitlow M. B., Iida K., Stefanova I., Bernard A., Nussenzweig V. H19, a surface membrane molecule involved in T-cell activation, inhibits channel formation by human complement. Cell Immunol. 1990 Mar;126(1):176–184. doi: 10.1016/0008-8749(90)90310-n. [DOI] [PubMed] [Google Scholar]