Abstract
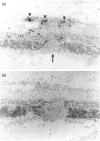
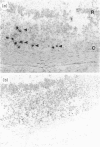
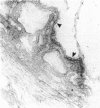
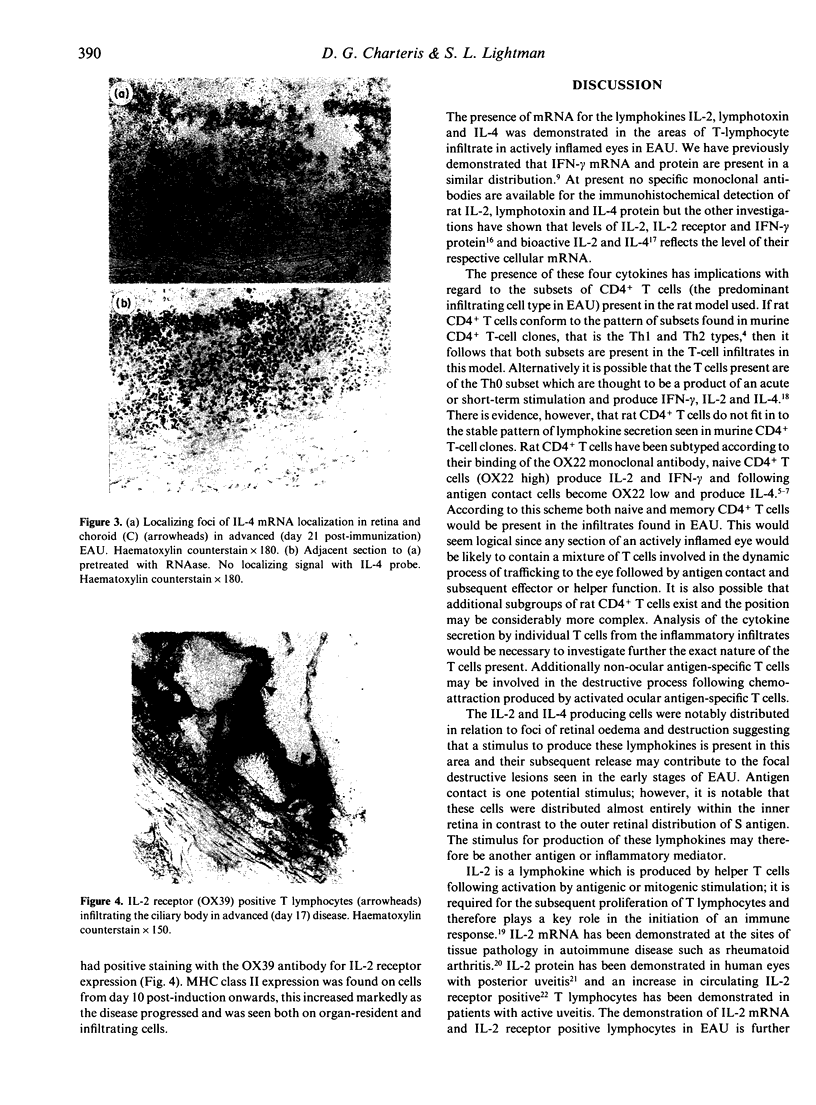
Experimental autoimmune uveoretinitis (EAU) is a well-characterized model of immune-mediated intraocular inflammation. The intraocular infiltrate in EAU consists predominantly of T lymphocytes. The in vivo production of interleukin-2 (IL-2), lymphotoxin and IL-4 by these T cells was investigated by in situ hybridization using cDNA probes to lymphokine mRNA. Localization of lymphokine mRNA was found simultaneous with disease onset in areas of T-cell infiltration. Positive signal was seen over cells in the uveal tract, retina and extraocular region. Less than 10% of the population of T cells defined immunohistochemically had positive localization of mRNA for these lymphokines. The number of positive cells was similar for each of the three probes and increased as the disease progressed. The findings suggest that these lymphokines are produced in vivo in immune-mediated intraocular inflammation and may play a role in the immunopathology seen in these conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carding S. R., West J., Woods A., Bottomly K. Differential activation of cytokine genes in normal CD4-bearing T cells is stimulus dependent. Eur J Immunol. 1989 Feb;19(2):231–238. doi: 10.1002/eji.1830190203. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Nussenblatt R. B., Palestine A. G., McAllister C., Gery I., BenEzra D. T-lymphocyte subsets in experimental autoimmune uveitis. Clin Immunol Immunopathol. 1985 Apr;35(1):103–110. doi: 10.1016/0090-1229(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Charteris D. G., Lightman S. L. Interferon-gamma (IFN-gamma) production in vivo in experimental autoimmune uveoretinitis. Immunology. 1992 Mar;75(3):463–467. [PMC free article] [PubMed] [Google Scholar]
- Deschênes J., Char D. H., Kaleta S. Activated T lymphocytes in uveitis. Br J Ophthalmol. 1988 Feb;72(2):83–87. doi: 10.1136/bjo.72.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey C., Cozette J., Faure J. P. A simple and rapid method for isolation of retinal S antigen. Ophthalmic Res. 1982;14(4):249–255. doi: 10.1159/000265199. [DOI] [PubMed] [Google Scholar]
- Fanslow W. C., Sims J. E., Sassenfeld H., Morrissey P. J., Gillis S., Dower S. K., Widmer M. B. Regulation of alloreactivity in vivo by a soluble form of the interleukin-1 receptor. Science. 1990 May 11;248(4956):739–742. doi: 10.1126/science.2139736. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Hooks J. J., Chan C. C., Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988 Sep;29(9):1444–1451. [PubMed] [Google Scholar]
- Jongeneel C. V., Nedospasov S. A., Plaetinck G., Naquet P., Cerottini J. C. Expression of the tumor necrosis factor locus is not necessary for the cytolytic activity of T lymphocytes. J Immunol. 1988 Mar 15;140(6):1916–1922. [PubMed] [Google Scholar]
- Lancki D. W., Hsieh C. S., Fitch F. W. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991 May 1;146(9):3242–3249. [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Lightman S., Greenwood J. Effect of lymphocytic infiltration on the blood-retinal barrier in experimental autoimmune uveoretinitis. Clin Exp Immunol. 1992 Jun;88(3):473–477. doi: 10.1111/j.1365-2249.1992.tb06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Barclay A. N., Mason D. W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991 May;21(5):1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Mason D. W., Barclay A. N. Sequence of rat interleukin 2 and anomalous binding of a mouse interleukin 2 cDNA probe to rat MHC class II-associated invariant chain mRNA. Immunogenetics. 1989;30(2):145–147. doi: 10.1007/BF02421547. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Paul N. L., Ruddle N. H. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Petty R. E., Johnston W., McCormick A. Q., Hunt D. W., Rootman J., Rollins D. F. Uveitis and arthritis induced by adjuvant: clinical, immunologic and histologic characteristics. J Rheumatol. 1989 Apr;16(4):499–505. [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Tite J. P. Differential requirement for protein synthesis in cytolysis mediated by class I and class II MHC-restricted cytotoxic T cells. Immunology. 1990 Aug;70(4):440–445. [PMC free article] [PubMed] [Google Scholar]
- Tite J. P. Evidence of a role for TNF-alpha in cytolysis by CD4+, class II MHC-restricted cytotoxic T cells. Immunology. 1990 Oct;71(2):208–212. [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Powell M. B., Ruddle N. H. Protein-antigen specific Ia-restricted cytolytic T cells: analysis of frequency, target cell susceptibility, and mechanism of cytolysis. J Immunol. 1985 Jul;135(1):25–33. [PubMed] [Google Scholar]
- WAKSMAN B. H., BULLINGTON S. J. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. III. Lesions of the eye. Arch Ophthalmol. 1960 Nov;64:751–762. doi: 10.1001/archopht.1960.01840010753018. [DOI] [PubMed] [Google Scholar]
- Warren C. J., Howell W. M., Bhambhani M., Cawley M. I., Smith J. L. An investigation of T-cell subset phenotype and function in the rheumatoid synovium using in situ hybridization for IL-2 mRNA. Immunology. 1991 Feb;72(2):250–255. [PMC free article] [PubMed] [Google Scholar]