Abstract
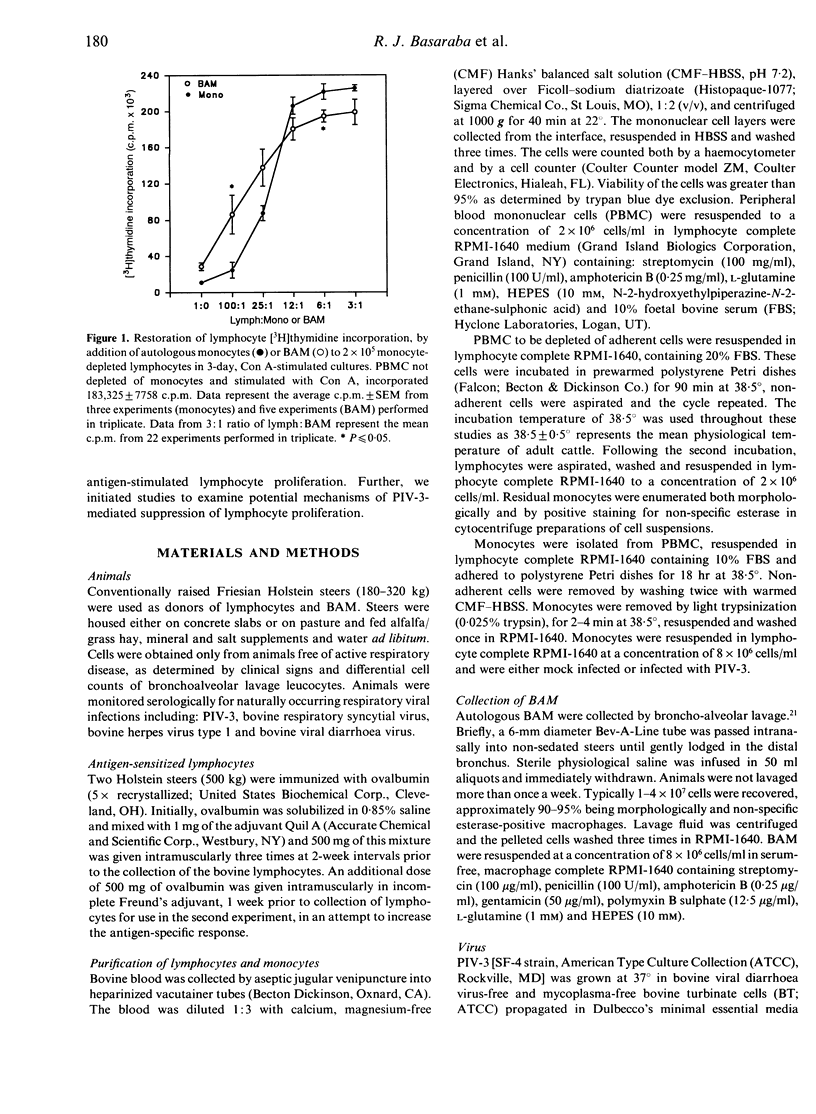
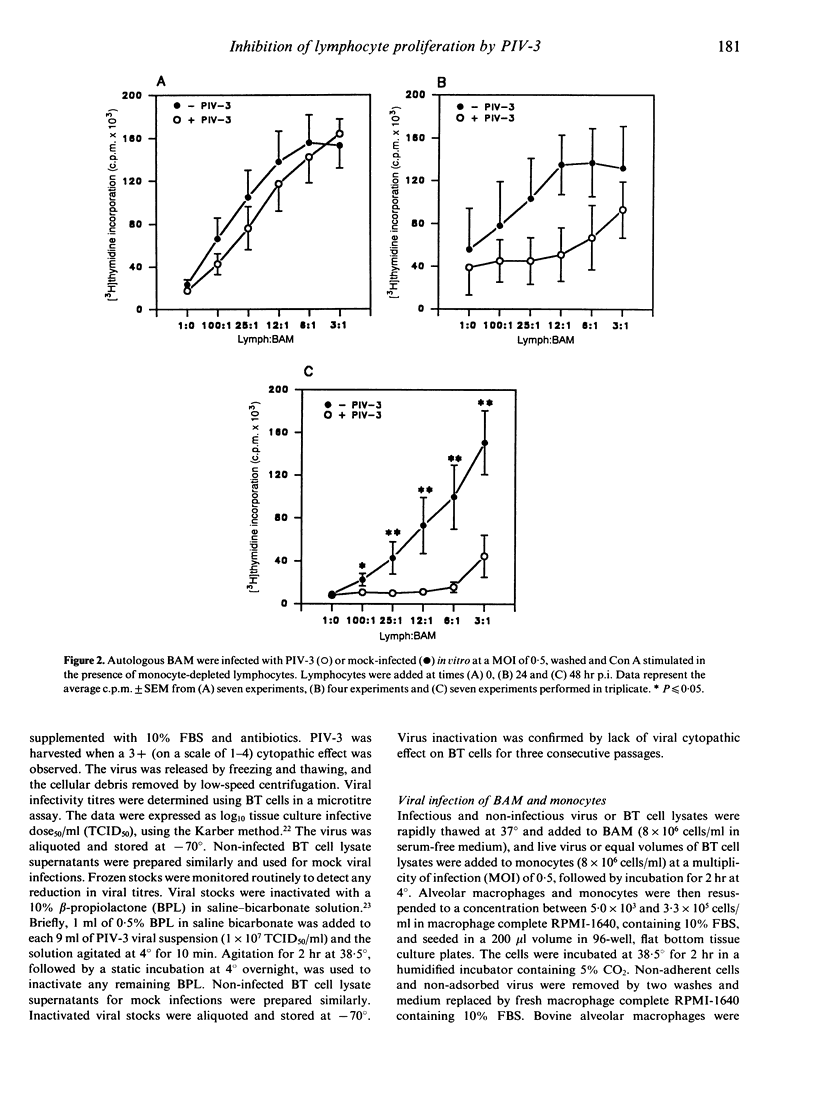
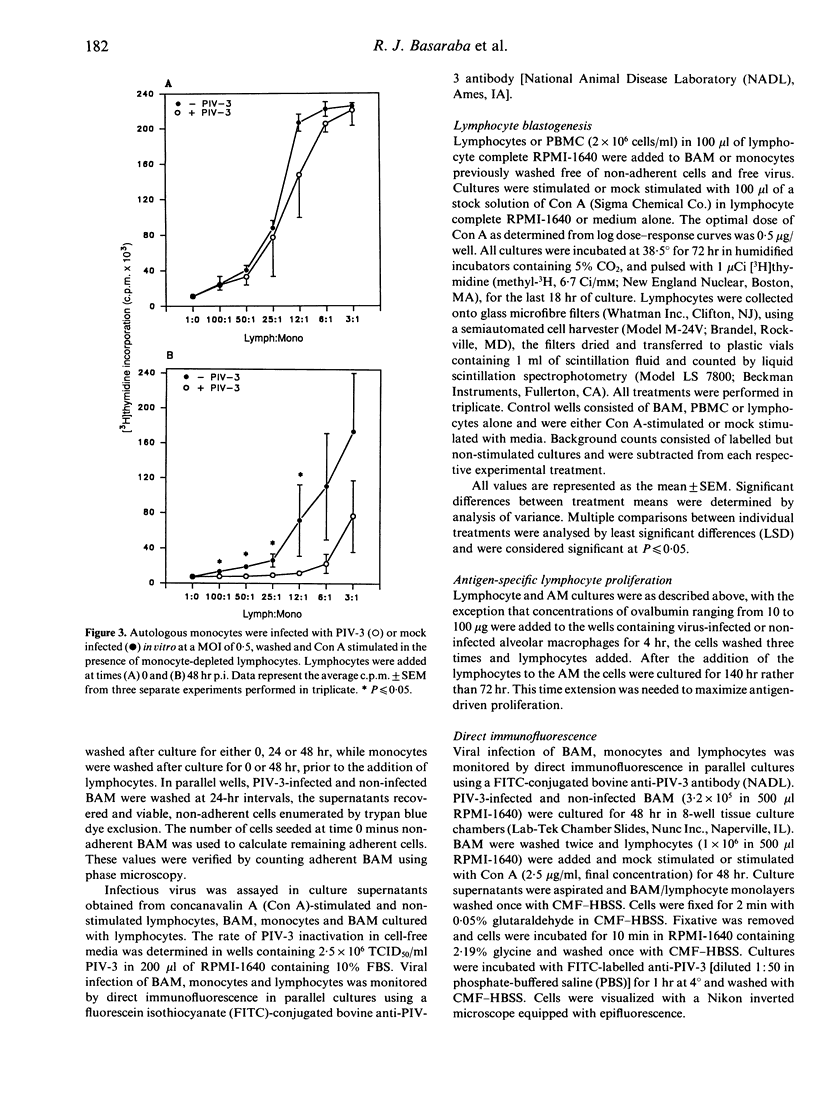
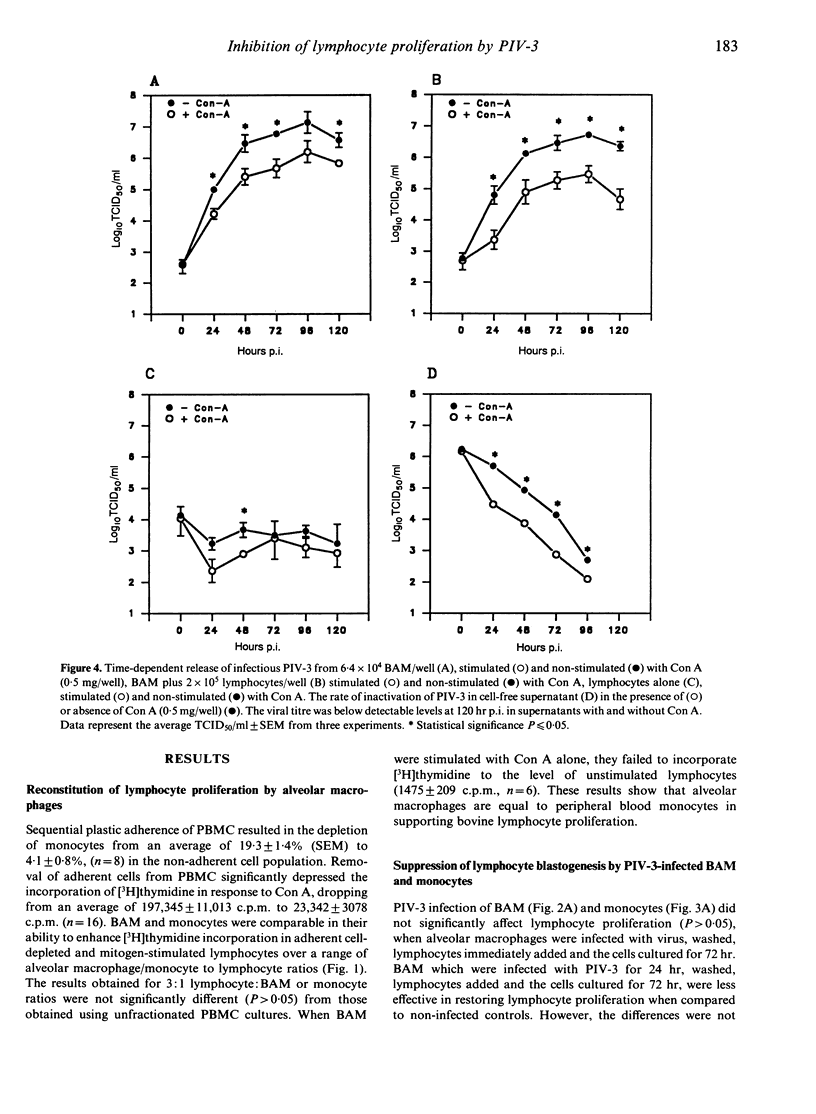
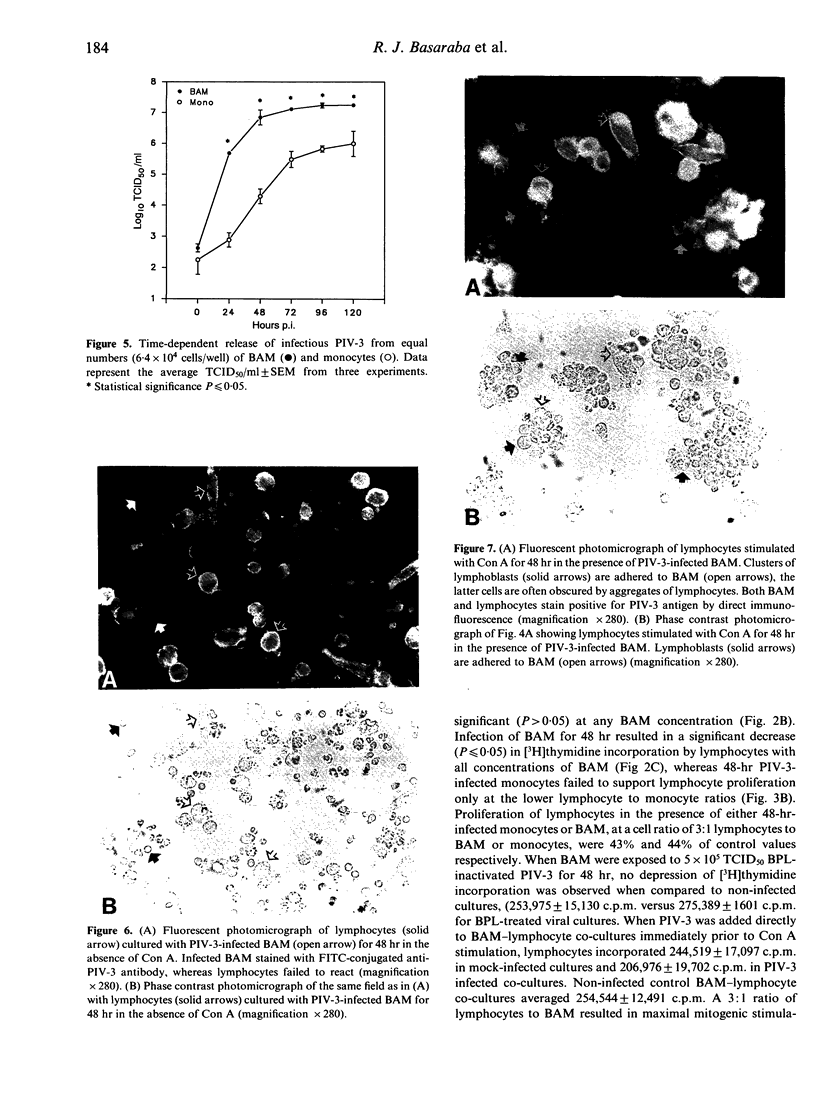
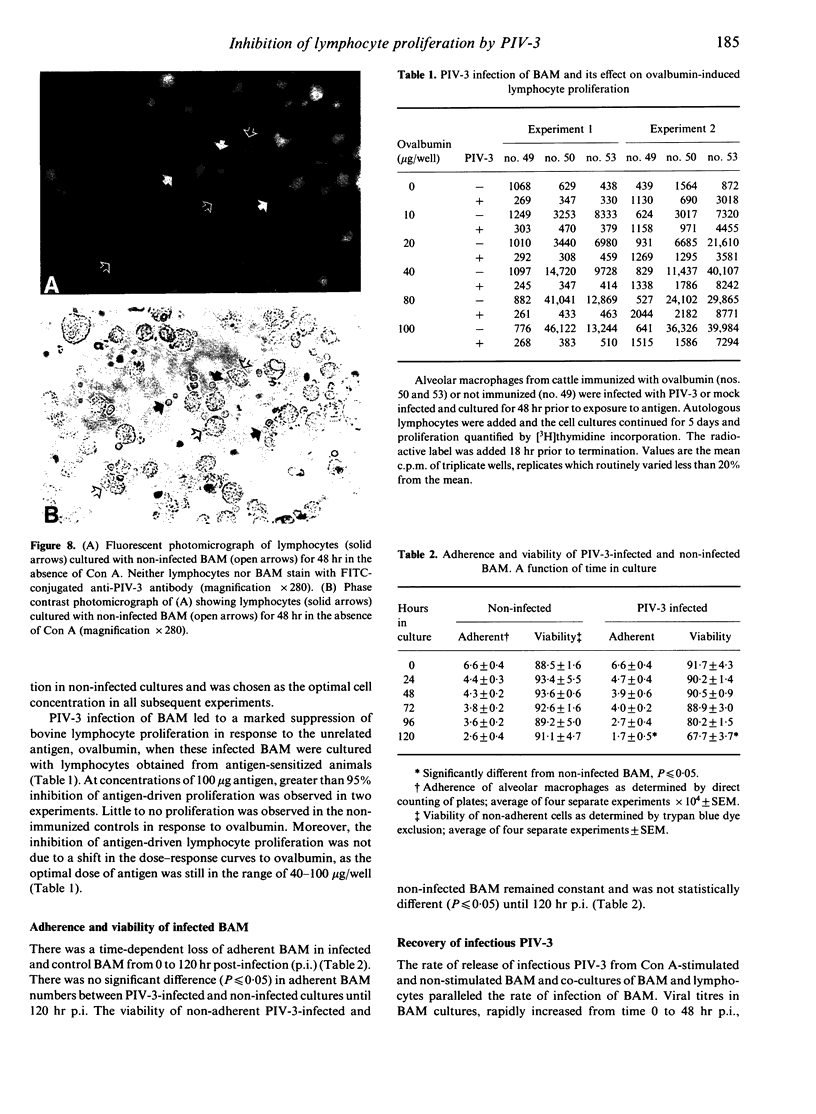
Lymphocytes stimulated with concanavalin A (Con A) or antigen in the presence of bovine parainfluenza virus type 3 (PIV-3) infected bovine alveolar macrophages (BAM) or monocytes, had depressed [3H]thymidine incorporation. This failure of lymphocytes to incorporate radiolabel required live virus, was time dependent and was most pronounced when BAM were infected for 48 hr prior to the addition of lymphocytes. The rate of infection of alveolar macrophages and the release of infectious virus into culture supernatants paralleled suppression of lymphocyte mitogenesis by PIV-3. However, the peak titre of exogenous, live or inactivated virus was not suppressive when added to lymphocyte macrophage cultures just prior to Con A stimulation. Neither the loss of viable alveolar macrophages nor a shift in antigen or mitogen dose response in virally infected cultures could account for the deficit in [3H]thymidine incorporation by lymphocytes. Despite the presence of lymphocyte-associated virus antigen detected by direct immunofluorescence, no increase in PIV-3 titre above baseline was seen from infected lymphocytes, irrespective of mitogen stimulation. Likewise, lymphocytes did not contribute to the extracellular virus pool in lymphocyte-macrophage cultures as the increases in viral titre above basal levels in supernatants were equal to levels released by macrophages alone. The expression of viral antigen on lymphocytes stimulated in the presence of PIV-3-infected BAM suggests a non-productive or abortive infection of lymphocytes mediated through contact with infected macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair B. M., Bradford H. E., Mackie D. P., McNulty M. S. Effect of macrophages and in vitro infection with parainfluenza type 3 and respiratory syncytial viruses on the mitogenic response of bovine lymphocytes. Am J Vet Res. 1992 Feb;53(2):225–229. [PubMed] [Google Scholar]
- Bryson D. G., McNulty M. S., McCracken R. M., Cush P. F. Ultrastructural features of experimental parainfluenza type 3 virus pneumonia in calves. J Comp Pathol. 1983 Jul;93(3):397–414. doi: 10.1016/0021-9975(83)90027-0. [DOI] [PubMed] [Google Scholar]
- Del Buono B. J., White S. M., Williamson P. L., Schlegel R. A. Plasma membrane lipid organization and the adherence of differentiating lymphocytes to macrophages. J Cell Physiol. 1989 Jan;138(1):61–69. doi: 10.1002/jcp.1041380110. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- Frank A. L., Taber L. H., Wells C. R., Wells J. M., Glezen W. P., Paredes A. Patterns of shedding of myxoviruses and paramyxoviruses in children. J Infect Dis. 1981 Nov;144(5):433–441. doi: 10.1093/infdis/144.5.433. [DOI] [PubMed] [Google Scholar]
- Ghram A., Reddy P. G., Blecha F., Minocha H. C. Effects of bovine respiratory disease viruses and isoprinosine on bovine leukocyte function in vitro. Vet Microbiol. 1989 Aug;20(4):307–314. doi: 10.1016/0378-1135(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Ghram A., Reddy P. G., Morrill J. L., Blecha F., Minocha H. C. Bovine herpesvirus-1 and parainfluenza-3 virus interactions: clinical and immunological response in calves. Can J Vet Res. 1989 Jan;53(1):62–67. [PMC free article] [PubMed] [Google Scholar]
- Giles R. E., Ruddle F. H. Production of Sendai virus for cell fusion. In Vitro. 1973 Sep-Oct;9(2):103–107. doi: 10.1007/BF02616007. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T., Tamashiro V. G., Moench T. R., Jauregui E., Lindo de Soriano I., Vaisberg A. In vitro studies of the role of monocytes in the immunosuppression associated with natural measles virus infections. Clin Immunol Immunopathol. 1987 Dec;45(3):375–383. doi: 10.1016/0090-1229(87)90090-0. [DOI] [PubMed] [Google Scholar]
- Hesse R. A., Toth T. E. Effects of bovine parainfluenza-3 virus on phagocytosis and phagosome-lysosome fusion of cultured bovine alveolar macrophages. Am J Vet Res. 1983 Oct;44(10):1901–1907. [PubMed] [Google Scholar]
- Härfast B., Andersson T., Stejskal V., Perlmann P. Interactions between human lymphocytes and paramyxovirus-infected cells: adsorption and cytotoxicity. J Immunol. 1977 Apr;118(4):1132–1137. [PubMed] [Google Scholar]
- Kantzler G. B., Lauteria S. F., Cusumano C. L., Lee J. D., Ganguly R., Waldman R. H. Immunosuppression during influenza virus infection. Infect Immun. 1974 Nov;10(5):996–1002. doi: 10.1128/iai.10.5.996-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O., Hirsch R. L. Immunosuppression in goats inoculated with parainfluenza type 3 virus. Am J Vet Res. 1983 Dec;44(12):2302–2306. [PubMed] [Google Scholar]
- Laegreid W. W., Taylor S. M., Leid R. W., Silflow R. M., Evermann J. R., Breeze R. G., Liggitt H. D. Virus-induced enhancement of arachidonate metabolism by bovine alveolar macrophages in vitro. J Leukoc Biol. 1989 Apr;45(4):283–292. doi: 10.1002/jlb.45.4.283. [DOI] [PubMed] [Google Scholar]
- Li X., Castleman W. L. Effects of 4-ipomeanol on bovine parainfluenza type 3 virus-induced pneumonia in calves. Vet Pathol. 1991 Sep;28(5):428–437. doi: 10.1177/030098589102800511. [DOI] [PubMed] [Google Scholar]
- Liggitt D., Huston L., Silflow R., Evermann J., Trigo E. Impaired function of bovine alveolar macrophages infected with parainfluenza-3 virus. Am J Vet Res. 1985 Aug;46(8):1740–1744. [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P. Abrogation of lymphocyte blastogenesis by a feline leukaemia virus protein. Nature. 1978 Aug 17;274(5672):687–689. doi: 10.1038/274687a0. [DOI] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oghiso Y. Heterogeneity in immunologic functions of rat alveolar macrophages--their accessory cell function and IL-1 production. Microbiol Immunol. 1987;31(3):247–260. doi: 10.1111/j.1348-0421.1987.tb03088.x. [DOI] [PubMed] [Google Scholar]
- Panigrahi P., Mohanty S. B., Maheshwari R. K., Friedman R. M. Structural proteins of bovine parainfluenza-3 virus. Vet Microbiol. 1987 Mar;13(3):205–210. doi: 10.1016/0378-1135(87)90083-6. [DOI] [PubMed] [Google Scholar]
- Poste G., Alexander D. J., Reeve P., Hewlett G. Modification of Newcastle disease virus release and cytopathogenicity in cells treated with plant lectins. J Gen Virol. 1974 Jun;23(3):255–270. doi: 10.1099/0022-1317-23-3-255. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr Different effects of influenza virus, respiratory syncytial virus, and Sendai virus on human lymphocytes and macrophages. Infect Immun. 1982 Mar;35(3):1142–1146. doi: 10.1128/iai.35.3.1142-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Rouse B. T., Horohov D. W. Immunosuppression in viral infections. Rev Infect Dis. 1986 Nov-Dec;8(6):850–873. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff D. M., Davies J., Desperbasques M., Billstrom M., Geerligs H. J., Welling G. W., Welling-Wester S., Buchan A., Skinner G. R. Immune inhibition of virus release from human and nonhuman cells by antibody to viral and host cell determinants. Intervirology. 1991;32(1):28–36. doi: 10.1159/000150182. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Ganz T. Host defense in pulmonary alveoli. Annu Rev Physiol. 1992;54:331–350. doi: 10.1146/annurev.ph.54.030192.001555. [DOI] [PubMed] [Google Scholar]
- Stauber E. H., Weston K. J. Association of parainfluenza-3 virus with bovine macrophages and blood cells: an in vitro study. Am J Vet Res. 1984 Mar;45(3):583–585. [PubMed] [Google Scholar]
- Trigo E., Liggitt H. D., Breeze R. G., Leid R. W., Silflow R. M. Bovine pulmonary alveolar macrophages: antemortem recovery and in vitro evaluation of bacterial phagocytosis and killing. Am J Vet Res. 1984 Sep;45(9):1842–1847. [PubMed] [Google Scholar]
- Tsai K. S. Replication of parainfluenza type 3 virus in alveolar macrophages: evidence of in vivo infection and of in vitro temperature sensitivity in virus maturation. Infect Immun. 1977 Dec;18(3):780–791. doi: 10.1128/iai.18.3.780-791.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Ogra P. L. Immunology of respiratory viral infections. Annu Rev Med. 1988;39:147–162. doi: 10.1146/annurev.me.39.020188.001051. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Wong D. T., Sun M., McCarthy N. Parainfluenza virus bronchiolitis. Epidemiology and pathogenesis. Am J Dis Child. 1986 Jan;140(1):34–40. doi: 10.1001/archpedi.1986.02140150036029. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Lymphocyte receptors for myxoviruses and paramyxoviruses. J Immunol. 1974 Jun;112(6):2176–2183. [PubMed] [Google Scholar]
- Yamamoto K., Inoue K., Suzuki K. Interaction of paramyxovirus with erythrocyte membranes modified by concanavalin A. Nature. 1974 Aug 9;250(5466):511–513. doi: 10.1038/250511a0. [DOI] [PubMed] [Google Scholar]
- Zisman B., Denman A. M. Inactivation of myxoviruses by lymphoid cells. J Gen Virol. 1973 Aug;20(2):211–223. doi: 10.1099/0022-1317-20-2-211. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., Hall S. L., London W. T., Kim H. W., Chanock R. M., Murphy B. R. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990 Aug;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]