Abstract
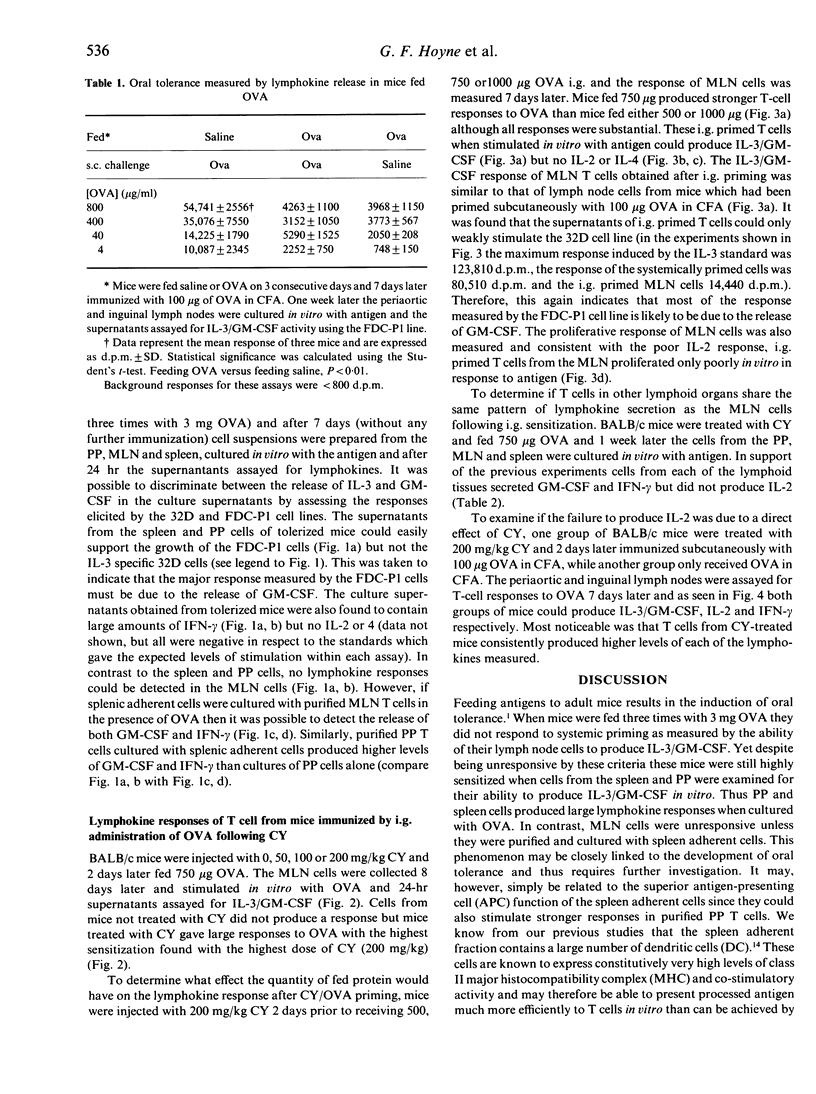
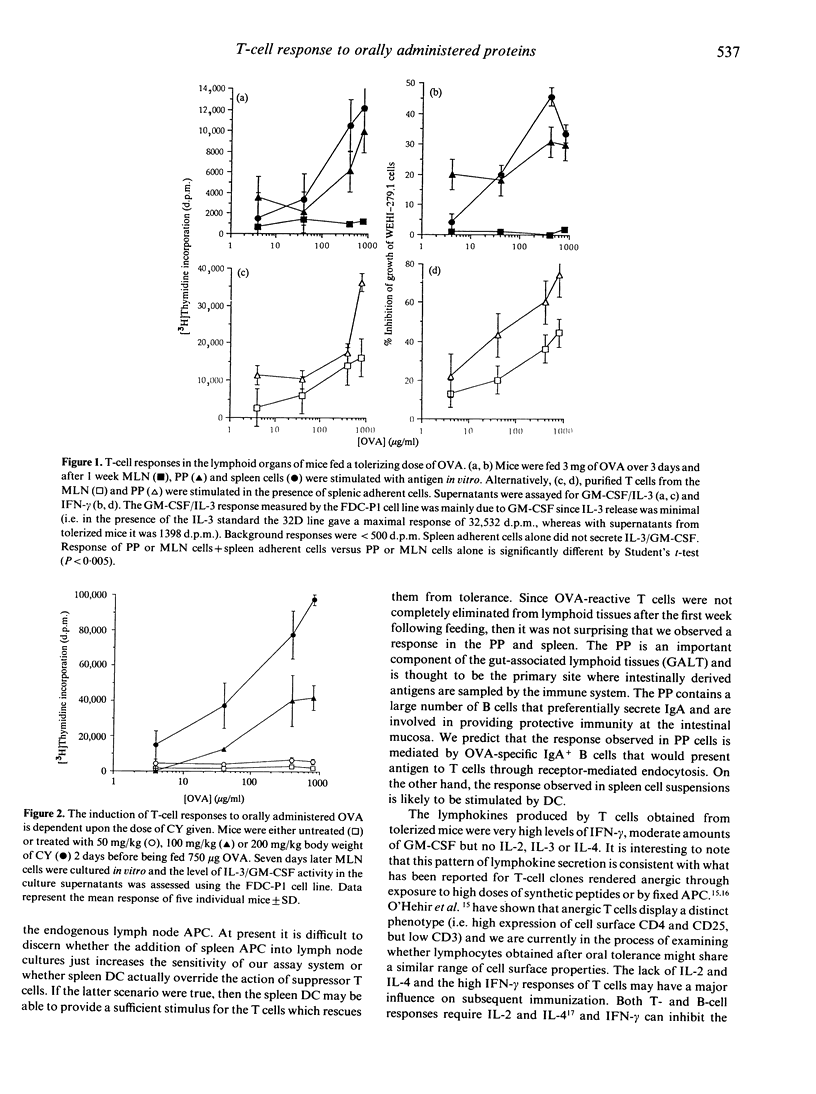
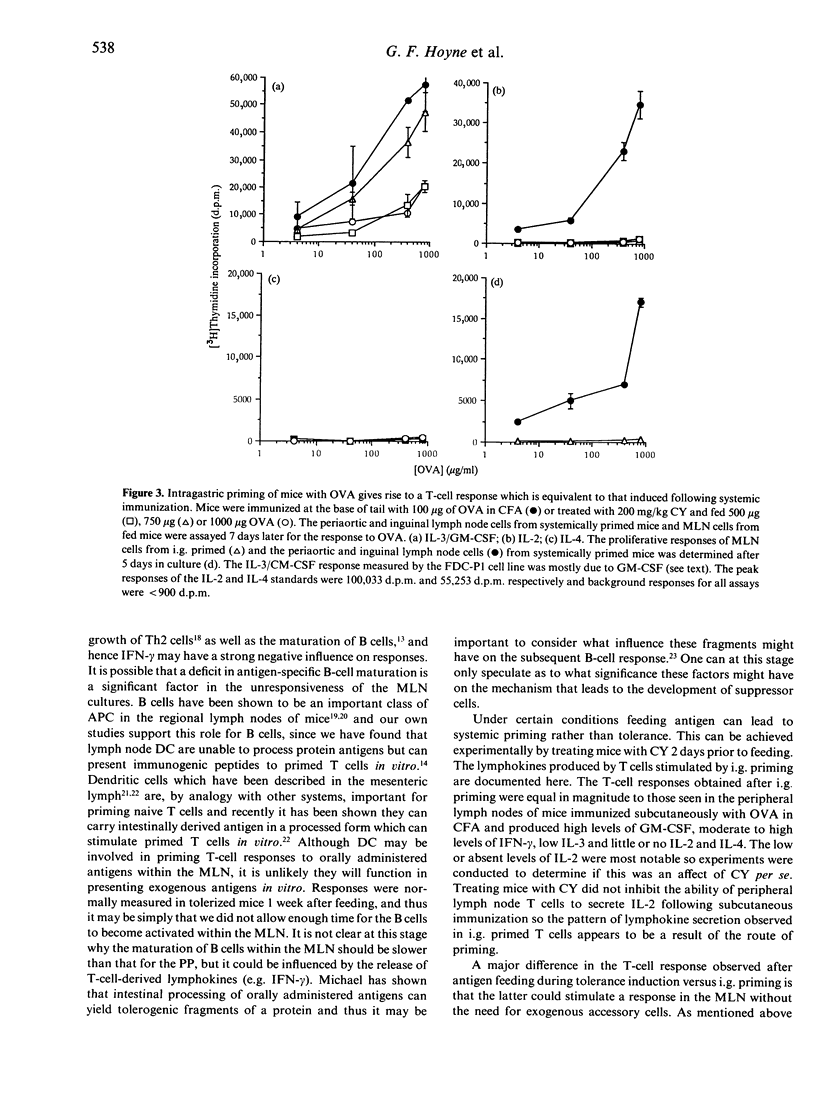
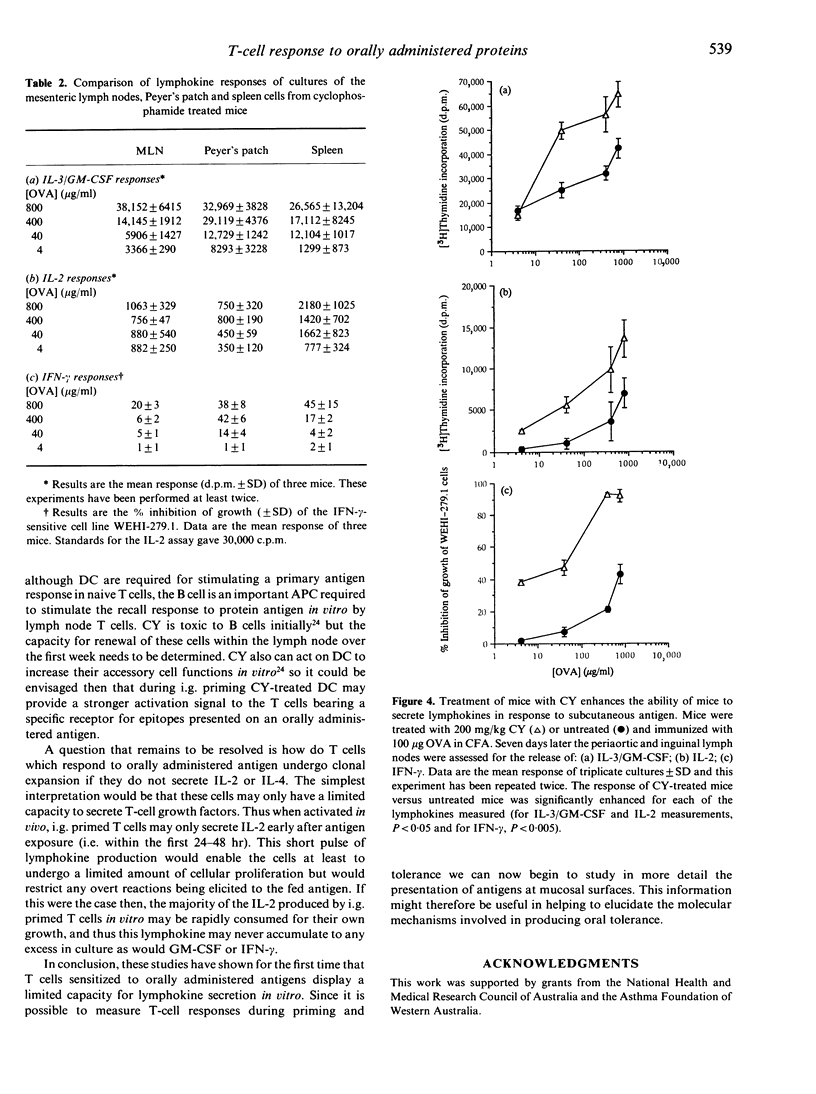
Feeding antigens induces an immunological unresponsiveness termed oral tolerance but under some conditions, for example following the administration of cyclophosphamide (CY), immunity can be induced. These observations have usually been made by studying antibody production and delayed hypersensitivity with little attention given to other measurements of cellular activation. We have therefore examined the lymphokines produced by T cells obtained after the induction of oral tolerance or intragastric priming. Cells isolated from the spleen and Peyer's patches (PP) of tolerized mice could secrete high levels of interferon-gamma (IFN-gamma) and moderate levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) in response to antigen while interleukin-2 (IL-2), IL-3 and IL-4 could not be detected. Mesenteric lymph node (MLN) cells of tolerized mice did not respond to antigen unless spleen adherent cells were added to the cultures where IFN-gamma and GM-CSF were produced. Intragastric priming was achieved by feeding antigen to CY-treated mice. T cells from the spleen, MLN and PP of these mice could produce GM-CSF, IFN-gamma, some IL-3 but little or no IL-2 and IL-4. The ability of MLN cells to proliferate with antigen in vitro was low and corresponded to low IL-2 production. Thus T cells from fed mice secrete a defined pattern of lymphokines which differs in tolerizing and priming regimes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Perera M. A., Thomas W. R. Contact sensitivity and the DNA response in mice to high and low doses of oxazolone: low dose unresponsiveness following painting and feeding and its prevention by pretreatment with cyclophosphamide. Immunology. 1979 Mar;36(3):449–459. [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M. Lymphoid dendritic cells. Immunology. 1987 Oct;62(2):161–170. [PMC free article] [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go C., Miller J. Differential induction of transcription factors that regulate the interleukin 2 gene during anergy induction and restimulation. J Exp Med. 1992 May 1;175(5):1327–1336. doi: 10.1084/jem.175.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Ron J., Katz M. E. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987 Feb 15;138(4):1051–1055. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Koike K., Ogawa M., Ihle J. N., Miyake T., Shimizu T., Miyajima A., Yokota T., Arai K. Recombinant murine granulocyte-macrophage (GM) colony-stimulating factor supports formation of GM and multipotential blast cell colonies in culture: comparison with the effects of interleukin-3. J Cell Physiol. 1987 Jun;131(3):458–464. doi: 10.1002/jcp.1041310319. [DOI] [PubMed] [Google Scholar]
- Limpens J., Van Meijer M., Van Santen H. M., Germeraad W. T., Hoeben-Schornagel K., Breel M., Scheper R. J., Kraal G. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991 Jul;73(3):255–263. [PMC free article] [PubMed] [Google Scholar]
- Liu L. M., MacPherson G. G. Lymph-borne (veiled) dendritic cells can acquire and present intestinally administered antigens. Immunology. 1991 Jul;73(3):281–286. [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- Michael J. G. The role of digestive enzymes in orally induced immune tolerance. Immunol Invest. 1989 Nov-Dec;18(9-10):1049–1054. doi: 10.3109/08820138909030606. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity in the small intestinal mucosa. V. Induction of cell-mediated immunity to a dietary antigen. Clin Exp Immunol. 1981 Mar;43(3):574–582. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Parrot D. M. Immunological responses to fed protein antigens in mice. IV. Effects of stimulating the reticuloendothelial system on oral tolerance and intestinal immunity to ovalbumin. Immunology. 1983 Dec;50(4):547–554. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M. The role of antigen recognition and suppressor cells in mice with oral tolerance to ovalbumin. Immunology. 1985 Oct;56(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- O'Hehir R. E., Yssel H., Verma S., de Vries J. E., Spits H., Lamb J. R. Clonal analysis of differential lymphokine production in peptide and superantigen induced T cell anergy. Int Immunol. 1991 Aug;3(8):819–826. doi: 10.1093/intimm/3.8.819. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Induction of IgE-secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985 Aug;94(1):182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]


