Abstract
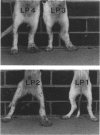
Recently, it has become possible to obtain as predicted disease manifestation in selectively bred dogs infected with the naturally occurring lymphatic nematode, Brugia pahangi. In this study, an attempt was made to correlate limb oedema with dynamic changes in immune cell responses occurring in the lymph node at the site of infection during onset of patency. Three litters of dogs were selectively bred; one for the expression of clinical disease, one for the lack of expression of clinical disease and one was of non-specific phenotype. Lymph node cells from 10 of 11 dogs showed a parasite-specific proliferative response at 4-6 weeks post-infection (p.i.), before the onset of patency. In six of 11 dogs, a loss of proliferative response to BpA in the infected node cells was detected around the time of onset of patency. In contrast, there was no reduction in the proliferative response to the mitogen phytohaemagglutinin (PHA). The proliferative response to BpA by unresponsive node cells could be restored by addition of substimulatory amounts of murine or human recombinant interleukin-2 (IL-2) to the culture medium. However, there was no correlation between the proliferative response of lymph node cells from infected limbs and the expression of clinical disease. Similarly, when in vitro parasite-specific antibody production by infected lymph node cells was examined, antibody production manifested by all dogs at 5 weeks p.i. was markedly changed at 8 weeks p.i., but these changes did not correlate with clinical disease. This lack of correlation indicates that the immune response to lymphatic filarial infection, as measured in this study, does not necessarily result directly in disease manifestation, and that other genetically determined factors may influence both the parasite-specific immune response and the clinical outcome of infection.
Full text
PDF




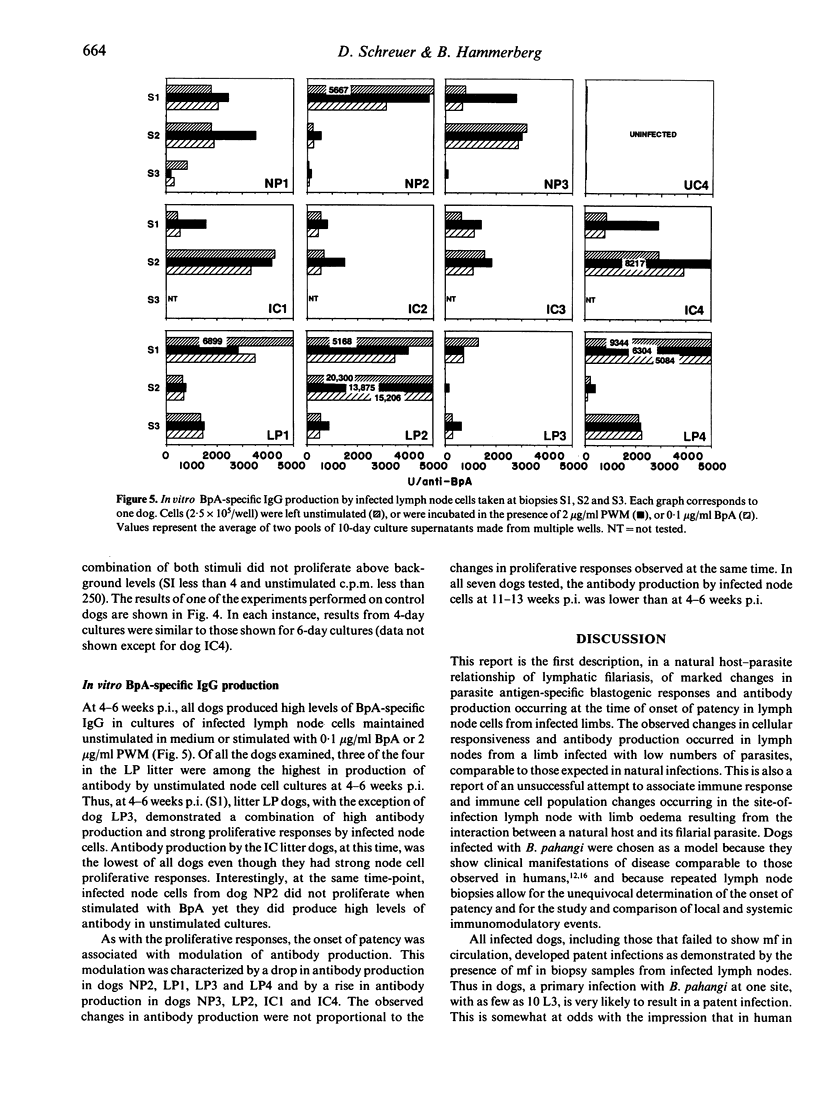


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley T. L., Brain S. D., Collins P. D., Williams T. J. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol. 1991 May 15;146(10):3424–3430. [PubMed] [Google Scholar]
- Denham D. A., Fletcher C. The cat infected with Brugia pahangi as a model of human filariasis. Ciba Found Symp. 1987;127:225–235. doi: 10.1002/9780470513446.ch15. [DOI] [PubMed] [Google Scholar]
- Denham D. A. Studies with Brugia pahangi. 6. The susceptibility of male and female cats to infection. J Parasitol. 1974 Aug;60(4):642–642. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Gebhard D. H., Carter P. B. Identification of canine T-lymphocyte subsets with monoclonal antibodies. Vet Immunol Immunopathol. 1992 Aug;33(3):187–199. doi: 10.1016/0165-2427(92)90181-o. [DOI] [PubMed] [Google Scholar]
- Hussain R., Hamilton R. G., Kumaraswami V., Adkinson N. F., Jr, Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981 Oct;127(4):1623–1629. [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. II. Qualitative characterization of filaria-specific IgE. J Immunol. 1983 Sep;131(3):1516–1521. [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. III. Specificities of IgE and IgG antibodies compared by immunoblot analysis. J Immunol. 1985 Aug;135(2):1415–1420. [PubMed] [Google Scholar]
- Kawakami Y., Custer M. C., Rosenberg S. A., Lotze M. T. IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol. 1989 May 15;142(10):3452–3461. [PubMed] [Google Scholar]
- King C. L., Ottesen E. A., Nutman T. B. Cytokine regulation of antigen-driven immunoglobulin production in filarial parasite infections in humans. J Clin Invest. 1990 Jun;85(6):1810–1815. doi: 10.1172/JCI114639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammie P. J., Katz S. P. Immunoregulation in experimental filariasis. II. Responses to parasite and nonparasite antigens in jirds with Brugia pahangi. J Immunol. 1983 Mar;130(3):1386–1389. [PubMed] [Google Scholar]
- Limaye A. P., Abrams J. S., Silver J. E., Ottesen E. A., Nutman T. B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990 Jul 1;172(1):399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Schreuer D., Hammerberg B. Inhibition of antigen-driven proliferative responses and enhancement of antibody production during infection with Brugia pahangi. J Immunol. 1991 Aug 1;147(3):1007–1013. [PubMed] [Google Scholar]
- Miller S., Snowden K., Schreuer D., Hammerberg B. Selective breeding of dogs for segregation of limb edema from microfilaremia as clinical manifestations of Brugia infections. Am J Trop Med Hyg. 1990 Nov;43(5):489–497. doi: 10.4269/ajtmh.1990.43.489. [DOI] [PubMed] [Google Scholar]
- Minakuchi R., Wacholtz M. C., Davis L. S., Lipsky P. E. Delineation of the mechanism of inhibition of human T cell activation by PGE2. J Immunol. 1990 Oct 15;145(8):2616–2625. [PubMed] [Google Scholar]
- Nutman T. B., Kumaraswami V., Pao L., Narayanan P. R., Ottesen E. A. An analysis of in vitro B cell immune responsiveness in human lymphatic filariasis. J Immunol. 1987 Jun 1;138(11):3954–3959. [PubMed] [Google Scholar]
- Ottesen E. A., Skvaril F., Tripathy S. P., Poindexter R. W., Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985 Apr;134(4):2707–2712. [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F., Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977 Sep;33(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M., Worms M. J., Maizels R. M., Ogilvie B. M. Rodent models of filariasis. Contemp Top Immunobiol. 1984;12:275–321. doi: 10.1007/978-1-4684-4571-8_8. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., McGreevy P. B., Piessens P. W., McGreevy M., Koiman I., Saroso J. S., Dennis D. T. Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980 Jan;65(1):172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Partono F., Hoffman S. L., Ratiwayanto S., Piessens P. W., Palmieri J. R., Koiman I., Dennis D. T., Carney W. P. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Engl J Med. 1982 Jul 15;307(3):144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Wadee A. A., Kurniawan L. Regulation of immune responses in lymphatic filariasis. Ciba Found Symp. 1987;127:164–179. doi: 10.1002/9780470513446.ch11. [DOI] [PubMed] [Google Scholar]
- Schacher J. F., Sahyoun P. F. A chronological study of the histopathology of filarial disease in cats and dogs caused by Brugia pahangi (Buckley and Edeson, 1956). Trans R Soc Trop Med Hyg. 1967;61(2):234–243. doi: 10.1016/0035-9203(67)90162-9. [DOI] [PubMed] [Google Scholar]
- Schacher J. F., Sulahian A., Edeson J. F. Experimental lymphoedema in dogs infected with Brugia spp. Trans R Soc Trop Med Hyg. 1969;63(5):682–684. doi: 10.1016/0035-9203(69)90195-3. [DOI] [PubMed] [Google Scholar]
- Snowden K. F., Hammerberg B. The lymphatic pathology of chronic Brugia pahangi infection in the dog. Trans R Soc Trop Med Hyg. 1989 Sep-Oct;83(5):670–678. doi: 10.1016/0035-9203(89)90394-5. [DOI] [PubMed] [Google Scholar]
- Snowden K., Hammerberg B. Dynamics of immune responses related to clinical status in Brugia pahangi-infected dogs. Am J Trop Med Hyg. 1987 Jul;37(1):143–151. doi: 10.4269/ajtmh.1987.37.143. [DOI] [PubMed] [Google Scholar]
- Ward D. J., Nutman T. B., Zea-Flores G., Portocarrero C., Lujan A., Ottesen E. A. Onchocerciasis and immunity in humans: enhanced T cell responsiveness to parasite antigen in putatively immune individuals. J Infect Dis. 1988 Mar;157(3):536–543. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Boros D. L. Changing patterns of lymphocyte proliferation, IL-2 production and utilization, and IL-2 receptor expression in mice infected with Schistosoma mansoni. J Immunol. 1990 Jul 15;145(2):724–731. [PubMed] [Google Scholar]