Abstract
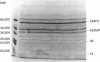
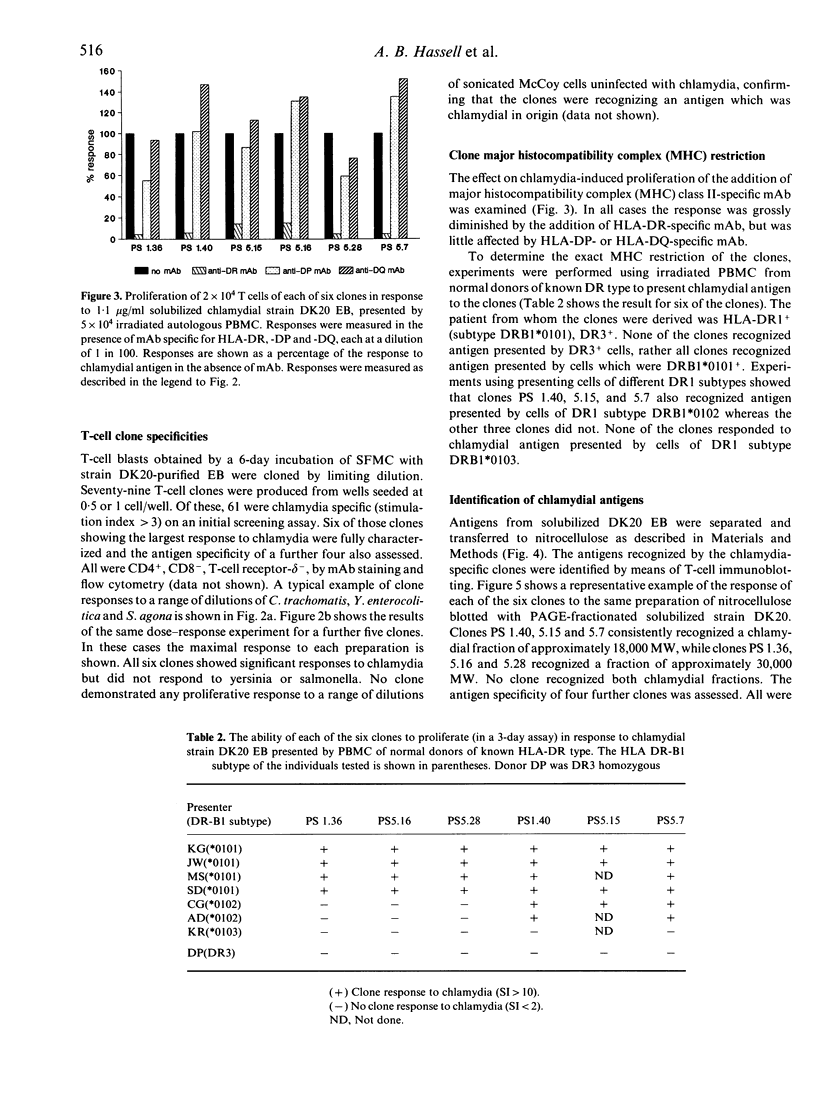
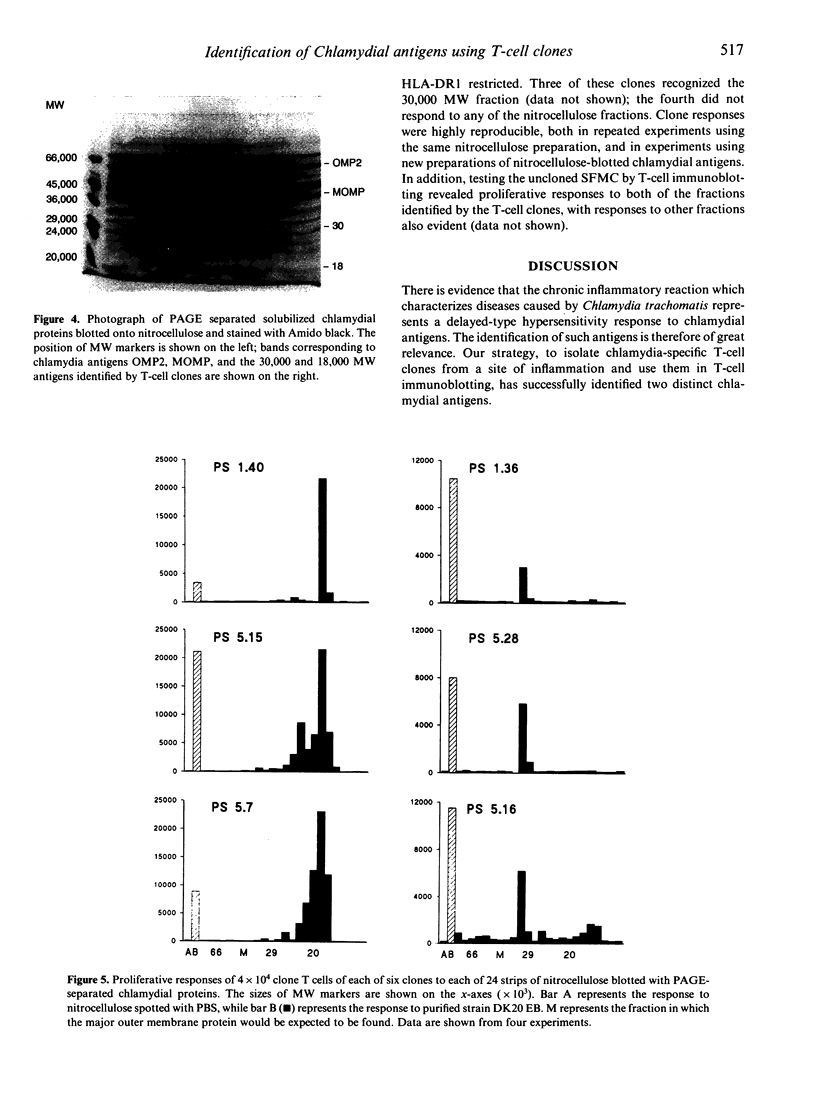
Chlamydia trachomatis is a major cause of sexually transmitted disease, infertility and reactive arthritis in the Western world, and of trachoma in the developing world. There is evidence that the chronic inflammatory reaction seen in diseases associated with chlamydiae represents a delayed-type hypersensitivity response to chlamydial antigens. Little is known about which chlamydial antigens elicit T-cell responses yet such information could have important implications in terms of both immunopathological understanding of these diseases and immunoprophylaxis design. In this study, 61 chlamydia-specific T-cell clones have been produced from the synovial fluid of an individual with sexually acquired reactive arthritis (SARA). Ten clones have been characterized in detail and used to identify T-cell stimulatory antigens of chlamydiae by means of T-cell immunoblotting. Two distinct antigenic fractions have been identified, one recognized by three of the clones (molecular weight 18,000), the other recognized by six of the clones (molecular weight 30,000). The fractions are distinct from the major outer membrane protein, the 57,000 MW stress protein and the 60,000 MW cysteine-rich membrane protein of chlamydiae. The major histocompatibility complex (MHC) restriction of the response to these antigens differed: clones recognizing the 18,000 MW antigen required antigen-presenting cells expressing DR1 subtype DRB1*0101 or DRB1*0102 which only differ at amino acids 85 and 86 on the DR beta-chain; by contrast clones recognizing the 30,000 MW antigen were presented to only by antigen-presenting cells from DRB1*0101 individuals, reflecting extreme sensitivity of these clones to the polymorphism at positions 85 and 86 on the DR beta-chain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Busch R., Hill C. M., Hayball J. D., Lamb J. R., Rothbard J. B. Effect of natural polymorphism at residue 86 of the HLA-DR beta chain on peptide binding. J Immunol. 1991 Aug 15;147(4):1292–1298. [PubMed] [Google Scholar]
- Coles A. M., Crosby H. A., Pearce J. H. Analysis of the human serological response to Chlamydia trachomatis 60-kDa proteins by two-dimensional electrophoresis and immunoblotting. FEMS Microbiol Lett. 1991 Jul 1;65(3):299–303. doi: 10.1016/0378-1097(91)90231-x. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Granfors K., Merilahti-Palo R., Bailey L., Consalvey S., Toivanen A., Bacon P. A. Synovial T lymphocyte recognition of organisms that trigger reactive arthritis. Clin Exp Immunol. 1989 Jun;76(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Hassell A. B., Pilling D., Reynolds D., Life P. F., Bacon P. A., Gaston J. S. MHC restriction of synovial fluid lymphocyte responses to the triggering organism in reactive arthritis. Absence of a class I-restricted response. Clin Exp Immunol. 1992 Jun;88(3):442–447. doi: 10.1111/j.1365-2249.1992.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. D., Johnston M. E., Chiu B., Falk J., Petric M. Immunochemical analysis of immune response to Chlamydia trachomatis in Reiter's syndrome and nonspecific urethritis. Clin Exp Immunol. 1987 Aug;69(2):246–254. [PMC free article] [PubMed] [Google Scholar]
- Keat A. C., Maini R. N., Pegrum G. D., Scott J. T. The clinical features and HLA associations of reactive arthritis associated with non-gonococcal urethritis. Q J Med. 1979 Apr;48(190):323–342. [PubMed] [Google Scholar]
- Keat A. C., Thomas B. J., Taylor-Robinson D., Pegrum G. D., Maini R. N., Scott J. T. Evidence of Chlamydia trachomatis infection in sexually acquired reactive arthritis. Ann Rheum Dis. 1980 Oct;39(5):431–437. doi: 10.1136/ard.39.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., O'Hehir R. E., Young D. B. The use of nitrocellulose immunoblots for the analysis of antigen recognition by T lymphocytes. J Immunol Methods. 1988 May 25;110(1):1–10. doi: 10.1016/0022-1759(88)90076-2. [DOI] [PubMed] [Google Scholar]
- Martin D. H., Pollock S., Kuo C. C., Wang S. P., Brunham R. C., Holmes K. K. Chlamydia trachomatis infections in men with Reiter's syndrome. Ann Intern Med. 1984 Feb;100(2):207–213. doi: 10.7326/0003-4819-100-2-207. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P. Chlamydial hsp60 and the immunopathogenesis of chlamydial disease. Semin Immunol. 1991 Jan;3(1):25–33. [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B., Willcox N., Wordsworth P., Beeson D., Vincent A., Altmann D., Lanchbury J. S., Harcourt G. C., Bell J. I., Newsom-Davis J. Critical role for the Val/Gly86 HLA-DR beta dimorphism in autoantigen presentation to human T cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7343–7347. doi: 10.1073/pnas.88.16.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J., Wølner-Hanssen P. Chlamydia trachomatis: a major threat to reproduction. Hum Reprod. 1989 Feb;4(2):111–124. doi: 10.1093/oxfordjournals.humrep.a136855. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schachter J., Hanna L., Hill E. C., Massad S., Sheppard C. W., Conte J. E., Jr, Cohen S. N., Meyer K. F. Are chlamydial infections the most prevalent venereal disease? JAMA. 1975 Mar 24;231(12):1252–1255. [PubMed] [Google Scholar]
- Schumacher H. R., Jr, Magge S., Cherian P. V., Sleckman J., Rothfuss S., Clayburne G., Sieck M. Light and electron microscopic studies on the synovial membrane in Reiter's syndrome. Immunocytochemical identification of chlamydial antigen in patients with early disease. Arthritis Rheum. 1988 Aug;31(8):937–946. doi: 10.1002/art.1780310801. [DOI] [PubMed] [Google Scholar]
- Sieper J., Kingsley G., Palacios-Boix A., Pitzalis C., Treharne J., Hughes R., Keat A., Panayi G. S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991 May;34(5):588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Schachter J., Caldwell H. D., Prendergast R. A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–3027. [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Treharne J. D., Murray A. Antigenic specificity of human antibody to chlamydia in trachoma and lymphogranuloma venereum. J Gen Microbiol. 1986 Jun;132(6):1599–1610. doi: 10.1099/00221287-132-6-1599. [DOI] [PubMed] [Google Scholar]
- Woolridge R. L., Grayston J. T., Chang I. H., Cheng K. H., Yang C. Y., Neave C. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol. 1967 May;63(5 Suppl):1645–1650. doi: 10.1016/0002-9394(67)94158-x. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]