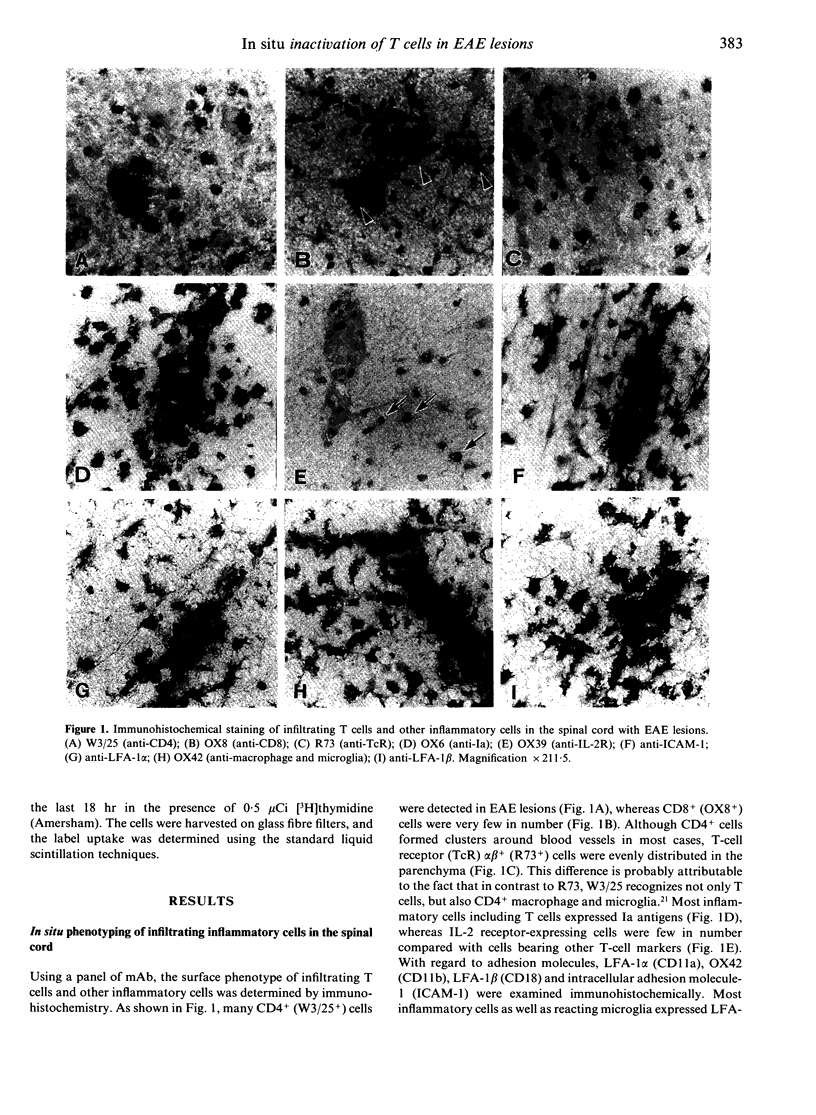
Abstract
Our previous study using bromodeoxyuridine (BrdU) has shown that T cells in lesions of experimental autoimmune encephalomyelitis (EAE) in the rat central nervous system (CNS) lose their proliferating capability immediately after infiltration into the CNS. To characterize the nature of this phenomenon in more detail, we have isolated T cells from EAE lesions and examined their surface phenotype and response to encephalitogenic antigen, myelin basic protein (MBP). By flow cytometry (FCM) analysis, it was revealed that compared with peripheral blood lymphocytes, up-regulation of interleukin-2 (IL-2) receptors (0.06%-->3.73%) and the lymphocyte function-associated antigen-1 (LFA-1) molecules (0.76%-->17.6%) on spinal cord T cells (SCT) was observed. In spite of the latter finding suggesting that SCT are activated, SCT recovered from rats with full-blown EAE responded very poorly to MBP. The addition of thymocytes or thymocytes plus astrocytes did not alter the low responsiveness of SCT. More importantly, astrocytes strongly suppressed the response of lymph node T cells to MBP. Using MBP-specific T-line cells, it was revealed that T-cell suppression might be induced by incomplete presentation of MBP and release of suppressive humoral factors by astrocytes. Since the response of SCT was still poor when assayed after three and 12 rounds of stimulation with the antigen and propagation with IL-2, this phenomenon is long lasting. These findings are consistent with the findings obtained by the BrdU study that infiltrating T cells into the CNS do not proliferate vigorously. Taken together, the poor response of infiltrating T cells to MBP would be induced by co-existing cells such as astrocytes although the T cells are in an active form as judged by their surface phenotype. The present study suggests that activation of non-haematopoietic parenchymal cells in each organ by infiltrating T cells and subsequent inactivation of the T cells are important healing processes for organ-specific autoimmune diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder T. A., Clark R. B., Goldschneider I. Relative susceptibility of SJL/J and B10.S mice to experimental allergic encephalomyelitis is correlated with high and low responsiveness to myelin basic protein. J Neuroimmunol. 1991 Dec;35(1-3):31–43. doi: 10.1016/0165-5728(91)90159-5. [DOI] [PubMed] [Google Scholar]
- Bodmer S., Strommer K., Frei K., Siepl C., de Tribolet N., Heid I., Fontana A. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989 Nov 15;143(10):3222–3229. [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., Nussenblatt R. B. Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science. 1987 Aug 28;237(4818):1029–1032. doi: 10.1126/science.2956685. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Essayan D. M., Zweiman B., Lisak R. P. Limiting dilution analysis of the frequency of antigen-reactive lymphocytes isolated from the central nervous system of Lewis rats with experimental allergic encephalomyelitis. Cell Immunol. 1987 Aug;108(1):203–213. doi: 10.1016/0008-8749(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Constam D. B., Philipp J., Malipiero U. V., ten Dijke P., Schachner M., Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992 Mar 1;148(5):1404–1410. [PubMed] [Google Scholar]
- Fallis R. J., Powers M. L., Sy M. S., Weiner H. L. Adoptive transfer of murine chronic-relapsing autoimmune encephalomyelitis. Analysis of basic protein-reactive cells in lymphoid organs and nervous system of donor and recipient animals. J Neuroimmunol. 1987 Mar;14(2):205–219. doi: 10.1016/0165-5728(87)90055-5. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Gaspari A. A., Jenkins M. K., Katz S. I. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988 Oct 1;141(7):2216–2220. [PubMed] [Google Scholar]
- Hayosh N. S., Swanborg R. H. Autoimmune effector cells. VII. Cells isolated from thymus and spinal cord of rats with experimental allergic encephalomyelitis transfer disease. Am J Pathol. 1986 Feb;122(2):218–222. [PMC free article] [PubMed] [Google Scholar]
- Hershkoviz R., Mor F., Gilat D., Cohen I. R., Lider O. T cells in the spinal cord in experimental autoimmune encephalomyelitis are matrix adherent and secrete tumor necrosis factor alpha. J Neuroimmunol. 1992 Mar;37(1-2):161–166. doi: 10.1016/0165-5728(92)90167-j. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Marquardt P., Cohen I. R. T lymphocyte lines induce autoimmune encephalomyelitis, delayed hypersensitivity and bystander encephalitis or arthritis. Eur J Immunol. 1984 Aug;14(8):729–734. doi: 10.1002/eji.1830140811. [DOI] [PubMed] [Google Scholar]
- Iwatani Y., Iitaka M., Row V. V., Volpé R. Effect of HLA-DR positive thyrocytes on in vitro thyroid autoantibody production. Clin Invest Med. 1988 Aug;11(4):279–285. [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Lyman W. D., Abrams G. A., Raine C. S. Experimental autoimmune encephalomyelitis: isolation and characterization of inflammatory cells from the central nervous system. J Neuroimmunol. 1989 Dec;25(2-3):195–201. doi: 10.1016/0165-5728(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Adoptively transferred experimental allergic encephalomyelitis in chimeric rats: identification of transferred cells in the lesions of the central nervous system. Immunology. 1988 Sep;65(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Matsumoto Y., Kawai K., Fujiwara M. Analysis of the T cell repertoire for myelin basic protein in thymus-grafted and other types of chimera: evidence that major histocompatibility complex molecules on accessory cells rather than T cell specificity mainly regulate susceptibility to autoimmune encephalomyelitis. Eur J Immunol. 1990 Sep;20(9):2119–2126. doi: 10.1002/eji.1830200934. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Kawai K., Fujiwara M. Hemorrhagic autoimmune encephalomyelitis induced by adoptive transfer of activated semiallogeneic spleen cells into irradiated rats. Am J Pathol. 1988 Nov;133(2):306–315. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Kawai K., Tomita Y., Fujiwara M. Limiting-dilution analysis of the frequency of myelin basic protein-reactive T cells in Lewis, PVG/c and BN rats. Implication for susceptibility to autoimmune encephalomyelitis. Immunology. 1990 Feb;69(2):215–221. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Ohmori K., Fujiwara M. Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology. 1992 Jun;76(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Ohmori K., Fujiwara M. Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J Neuroimmunol. 1992 Mar;37(1-2):23–33. doi: 10.1016/0165-5728(92)90152-b. [DOI] [PubMed] [Google Scholar]
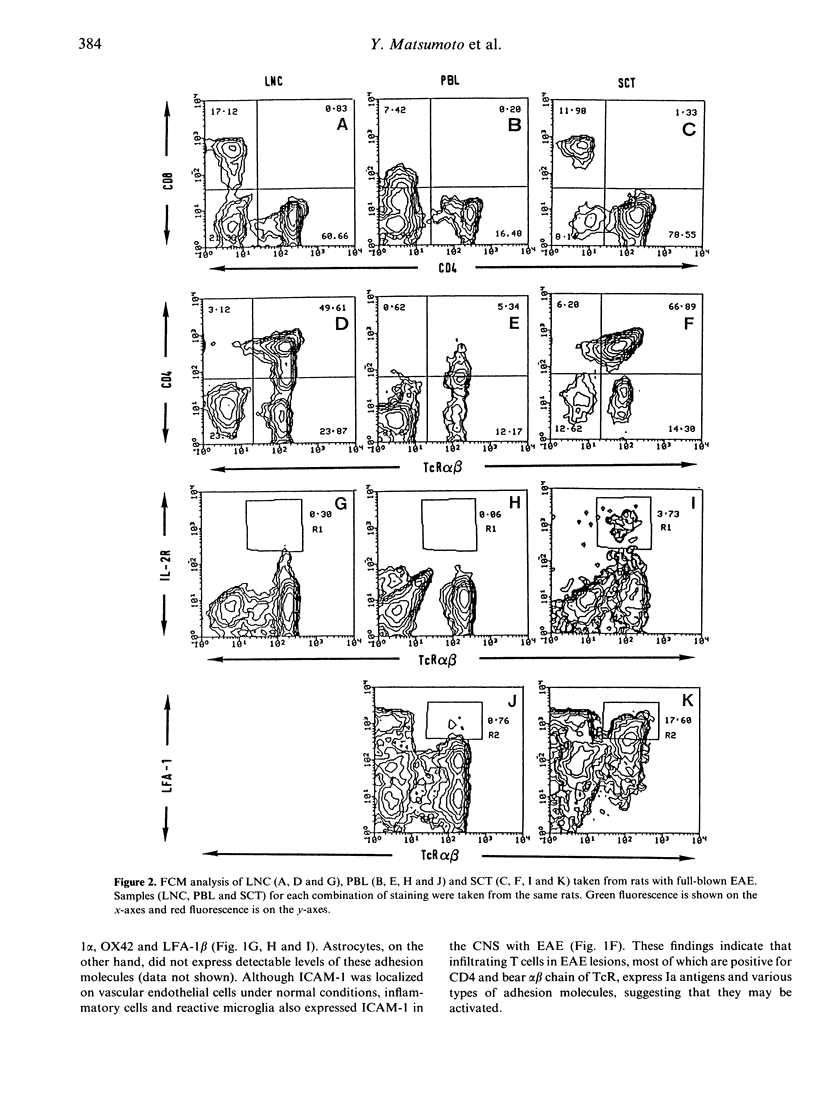
- Matsumoto Y., Watabe K., Ikuta F. Immunohistochemical study on neuroglia identified by the monoclonal antibody against a macrophage differentiation antigen (Mac-1). J Neuroimmunol. 1985 Oct;9(6):379–389. doi: 10.1016/s0165-5728(85)80037-0. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Ben-Nun A., Holoshitz J., Reshef T., Frenkel A., Rosenberg M., Cohen I. R. T lymphocyte lines producing or vaccinating against autoimmune encephalomyelitis (EAE). Functional activation induces peanut agglutinin receptors and accumulation in the brain and thymus of line cells. Eur J Immunol. 1983 May;13(5):418–423. doi: 10.1002/eji.1830130513. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Cannella B., Albert P., Sporn M., Raine C. S., McFarlin D. E. Evidence of endogenous regulatory function of transforming growth factor-beta 1 in experimental allergic encephalomyelitis. Int Immunol. 1992 May;4(5):615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
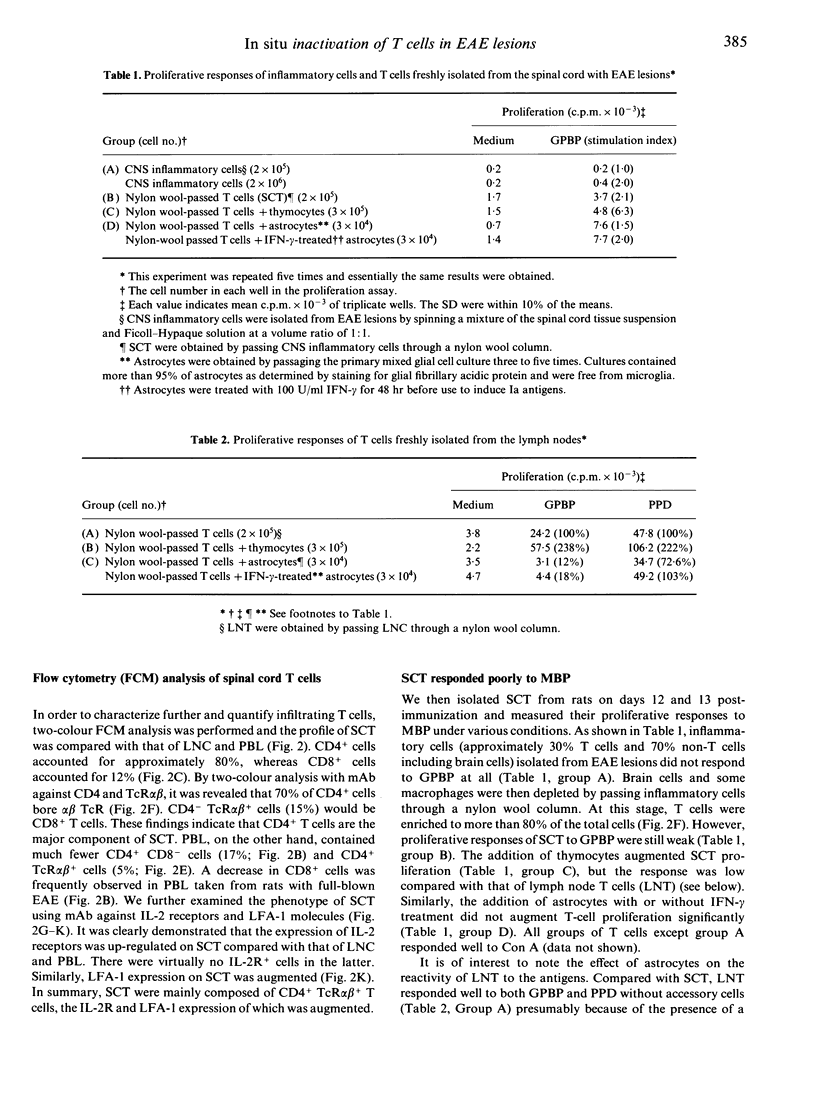
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- da Cunha A., Vitković L. Transforming growth factor-beta 1 (TGF-beta 1) expression and regulation in rat cortical astrocytes. J Neuroimmunol. 1992 Feb;36(2-3):157–169. doi: 10.1016/0165-5728(92)90047-o. [DOI] [PubMed] [Google Scholar]