Abstract
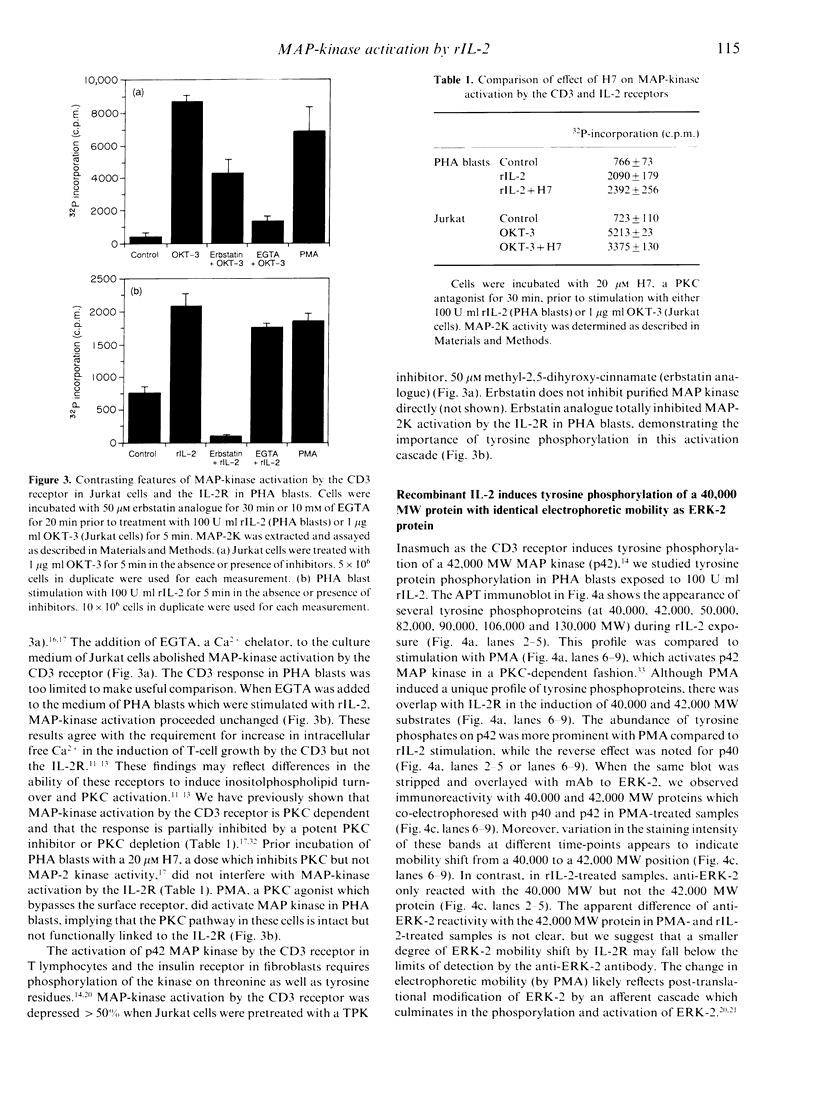
We investigated activation of mitogen-activated protein (MAP) kinase, also known as microtubule associated protein-2 kinase (MAP-2K), by recombinant interleukin-2 (rIL-2) in phytohaemagglutinin (PHA)-induced peripheral blood lymphoblasts (PBL). MAP-kinase activation has been implicated in growth of lymphocytes and other cell types. Enzyme activity was purified from cell lysates by ion-exchange chromatography and activity measured by the ability to phosphorylate the substrates MAP-2 and myelin basic protein peptide (APRTPGGRR) in vitro. Recombinant IL-2 stimulated a variable (two-to 10-fold) and evanescent MAP-2K response which was dose dependent over the range 0-50 U/ml. In contrast to MAP-kinase activation by the CD3 receptor, activation by the IL-2 receptor (IL-2R) proceeded independently from protein kinase C (PKC) and extracellular-free Ca2+. MAP-kinase activation by CD3 involves an activation cascade which depends on Ca2+ influx and PKC activation. These events culminate in tyrosine phosphorylation and activation of MAP kinase. Recombinant IL-2 induced tyrosine phosphorylation of several intracellular proteins, including a 40,000 MW substrate which co-electrophoresed with ERK-2 on SDS-PAGE. The ERK-2 gene encodes a 41,000 MW MAP-2K and is subject to regulation by a variety of mitogens and growth factors in lymphocytes and non-lymphoid cells. MAP-kinase activation by rIL-2 was abrogated when PHA blasts were pretreated with the tyrosine protein kinase (TPK) inhibitor, methyl-2,5-dihydroxy-cinnamate. Although the TPK, p56lck, has been implicated in the activation of MAP kinase and the function of IL-2R, we found no mobility shift from a 56,000 to a 60,000 MW position as seen during PKC activation. Together these data suggest that tyrosine phosphorylation is critical to IL-2-mediated signal transduction and that MAP kinase is one of the cellular intermediates involved in this pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima N., Kuziel W. A., Grdina T. A., Greene W. C. IL-2-induced signal transduction involves the activation of nuclear NF-kappa B expression. J Immunol. 1992 Jul 1;149(1):83–91. [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas A., Hanekom C., Williams K., Katz R., Nel A. E. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991 Oct 5;266(28):19088–19094. [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Ettehadieh E., Sanghera J. S., Pelech S. L., Hess-Bienz D., Watts J., Shastri N., Aebersold R. Tyrosyl phosphorylation and activation of MAP kinases by p56lck. Science. 1992 Feb 14;255(5046):853–855. doi: 10.1126/science.1311128. [DOI] [PubMed] [Google Scholar]
- Evans S. W., Farrar W. L. Interleukin 2 and diacylglycerol stimulate phosphorylation of 40 S ribosomal S6 protein. Correlation with increased protein synthesis and S6 kinase activation. J Biol Chem. 1987 Apr 5;262(10):4624–4630. [PubMed] [Google Scholar]
- Fung M. R., Scearce R. M., Hoffman J. A., Peffer N. J., Hammes S. R., Hosking J. B., Schmandt R., Kuziel W. A., Haynes B. F., Mills G. B. A tyrosine kinase physically associates with the beta-subunit of the human IL-2 receptor. J Immunol. 1991 Aug 15;147(4):1253–1260. [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hanekom C., Nel A., Gittinger C., Rheeder A., Landreth G. Complexing of the CD-3 subunit by a monoclonal antibody activates a microtubule-associated protein 2 (MAP-2) serine kinase in Jurkat cells. Biochem J. 1989 Sep 1;262(2):449–456. doi: 10.1042/bj2620449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Interleukin 2-induced lymphocyte proliferation is independent of increases in cytosolic-free calcium concentrations. J Immunol. 1985 Apr;134(4):2431–2435. [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Fung M., Baker M., Sutherland R., Greene W. C. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [PubMed] [Google Scholar]
- Mills G. B., Stewart D. J., Mellors A., Gelfand E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J Immunol. 1986 Apr 15;136(8):3019–3024. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Hultin L. Protein kinase C plays a role in the induction of tyrosine phosphorylation of lymphoid microtubule-associated protein-2 kinase. Evidence for a CD3-associated cascade that includes pp56lck and that is defective in HPB-ALL. J Immunol. 1991 Sep 15;147(6):1933–1939. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Nel A. E., Schabort I., Rheeder A., Bouic P., Wooten M. W. Inhibition of antibodies to CD3 surface antigen and phytohemagglutinin-mediated T cellular responses by inhibiting Ca2+/phospholipid-dependent protein kinase activity with the aid of 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine dihydrochloride. J Immunol. 1987 Oct 1;139(7):2230–2236. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Sabath D. E., Hoover R. G., Prystowsky M. B. Recombinant interleukin 2 regulates levels of c-myc mRNA in a cloned murine T lymphocyte. Mol Cell Biol. 1985 Dec;5(12):3361–3368. doi: 10.1128/mcb.5.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]




