Abstract
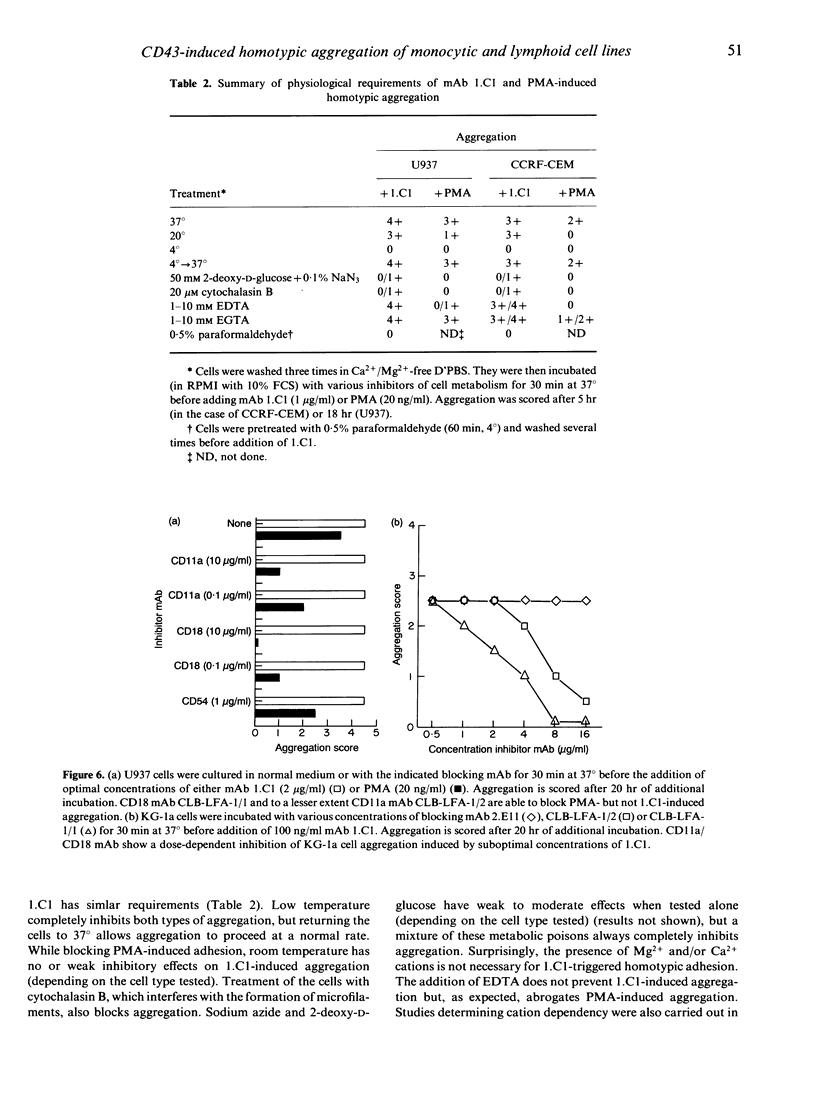
We describe a monoclonal antibody (mAb), designated 1.C1, that causes rapid and vigorous aggregation among normal leucocytes and among T and myeloid/monocytic cell lines. As shown by competitive binding and sequential immunoprecipitation experiments, the antigen recognized by mAb 1.C1 is a 115,000 MW sialoglycoprotein, that corresponds to the human CD43 antigen, also known as leukosialin or sialophorin. The aggregation process starts within minutes and reaches maximum level 6-18 hr after addition of the antibody. It is dependent on active cell metabolism (inhibited at low temperatures and by a mixture of the metabolic poisons azide and 2-deoxy-D-glucose), a fluid plasma membrane (inhibited by pretreatment of the cells with paraformaldehyde) and an intact cytoskeleton (inhibited by cytochalasin B). Two reference CD43 antibodies (MEM-59 and DF-T1), both binding the same or closely related sialic acid-dependent epitope as mAb 1.C1, are also capable of inducing cell clump formation. CD11a/CD18 mAb block the 1.C1-induced adhesion of resting peripheral blood leucocytes, but not of haematopoietic cell line cells. In addition, mAb 1.C1 induces homotypic aggregation of K-562 cells, which do not express members of the beta 2 integrin subfamily on their surface. These data suggest that triggering of the CD43 antigen promotes homotypic cell adhesion that is mediated by both CD11a/CD18-dependent and -independent pathways.
Full text
PDF
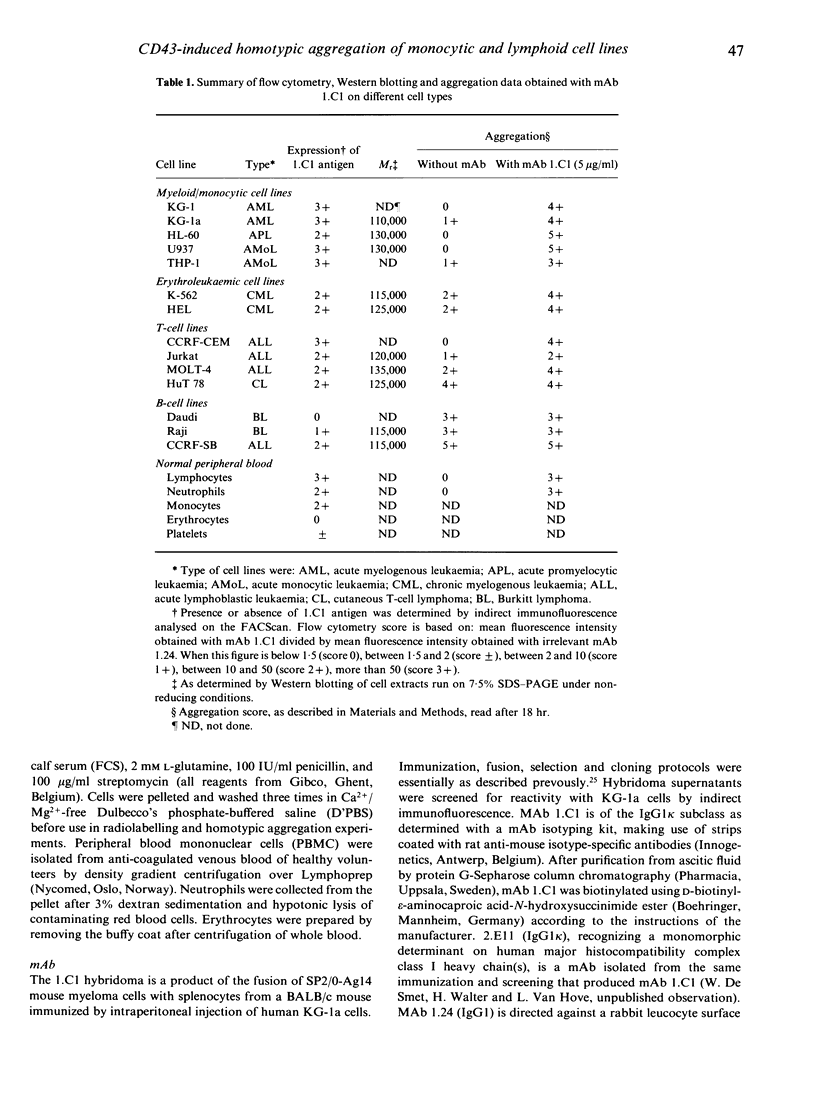
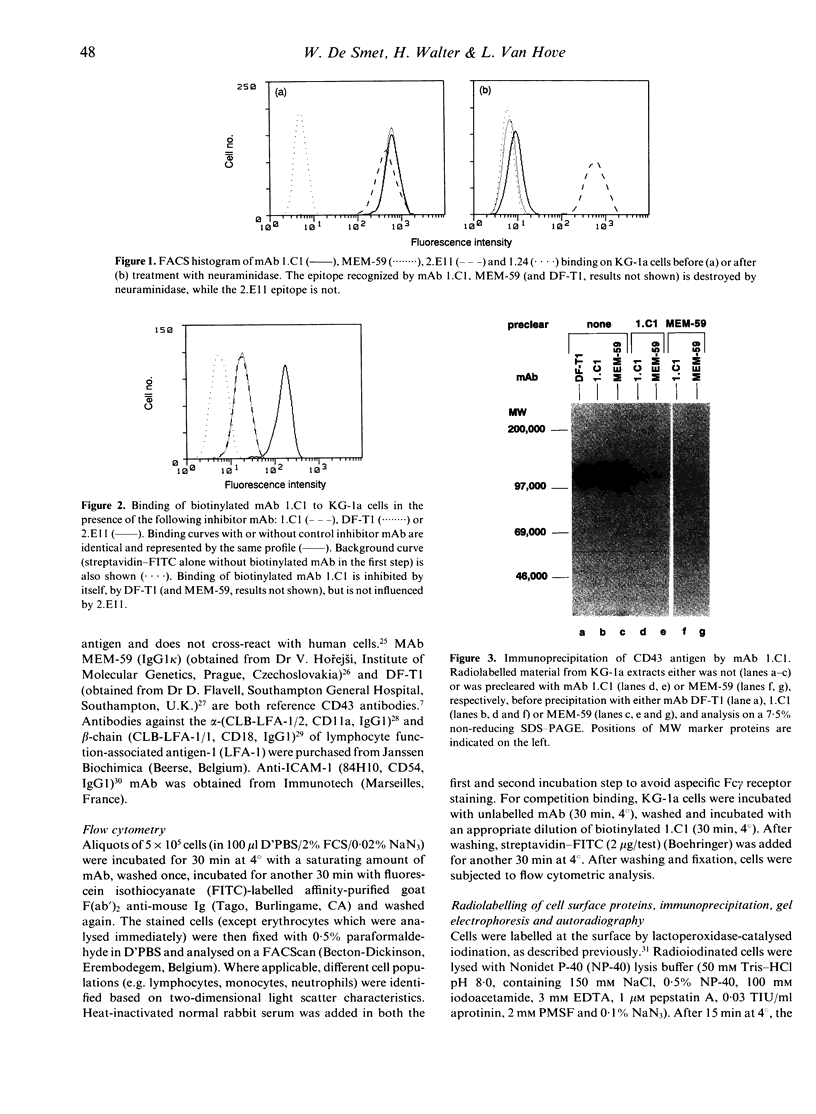

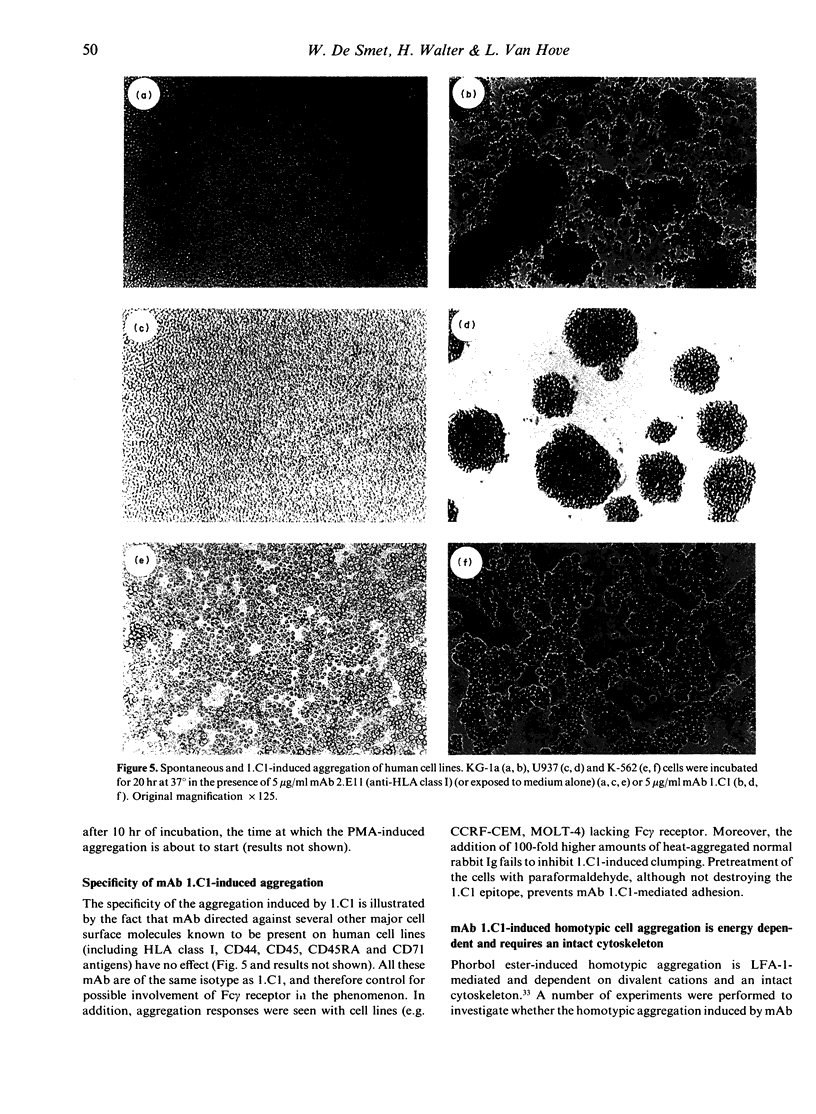



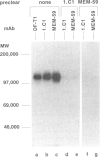
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardman B., Sikorski M. A., Staunton D. E. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson B., Hammarström S., Robertsson E. S., Aman P., Perlmann P., Mellstedt H. The large sialoglycoprotein of human lymphocytes. I. Distribution on T and B lineage cells as revealed by a monospecific chicken antibody. Eur J Immunol. 1985 May;15(5):417–426. doi: 10.1002/eji.1830150502. [DOI] [PubMed] [Google Scholar]
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Borche L., Lozano F., Vilella R., Vives J. CD43 monoclonal antibodies recognize the large sialoglycoprotein of human leukocytes. Eur J Immunol. 1987 Oct;17(10):1523–1526. doi: 10.1002/eji.1830171023. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991 Apr;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J. G., Williams A. F. The importance of cross-linking in the homotypic aggregation of lymphocytes induced by anti-leukosialin (CD43) antibodies. Eur J Immunol. 1992 Oct;22(10):2565–2572. doi: 10.1002/eji.1830221015. [DOI] [PubMed] [Google Scholar]
- Cyster J., Somoza C., Killeen N., Williams A. F. Protein sequence and gene structure for mouse leukosialin (CD43), a T lymphocyte mucin without introns in the coding sequence. Eur J Immunol. 1990 Apr;20(4):875–881. doi: 10.1002/eji.1830200424. [DOI] [PubMed] [Google Scholar]
- De Smet W., Vaeck M., Smet E., Brys L., Hamers R. Rabbit leukocyte surface antigens defined by monoclonal antibodies. Eur J Immunol. 1983 Nov;13(11):919–928. doi: 10.1002/eji.1830131111. [DOI] [PubMed] [Google Scholar]
- Dorfman K. S., Litaker W., Baecher C. M., Frelinger J. G. The nucleotide sequence of Ly 48 (mouse leukosialin, sialophorin): the mouse homolog of CD43. Nucleic Acids Res. 1990 Aug 25;18(16):4932–4932. doi: 10.1093/nar/18.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Autero M., Hermonen J. Major O-glycosylated sialoglycoproteins of human hematopoietic cells: differentiation antigens with poorly understood functions. J Cell Biochem. 1988 May;37(1):91–105. doi: 10.1002/jcb.240370109. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Cambier J. C., Tedder T. F. CD4 binding to major histocompatibility complex class II antigens induces LFA-1-dependent and -independent homotypic adhesion of B lymphocytes. Eur J Immunol. 1992 Jan;22(1):147–152. doi: 10.1002/eji.1830220122. [DOI] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Hoogerwerf M., Kuijpers K. C., Schwartz B. R., Harlan J. M. Cross-linking of sialophorin (CD43) induces neutrophil aggregation in a CD18-dependent and a CD18-independent way. J Immunol. 1992 Aug 1;149(3):998–1003. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Hesselink W. G., Werner G., Spits H., Melief C. J. Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol. 1984 Jun;14(6):518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- Nong Y. H., Remold-O'Donnell E., LeBien T. W., Remold H. G. A monoclonal antibody to sialophorin (CD43) induces homotypic adhesion and activation of human monocytes. J Exp Med. 1989 Jul 1;170(1):259–267. doi: 10.1084/jem.170.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K., Rosenstein Y. J., Remold-O'Donnell E., Bierer B. E., Rosen F. S., Burakoff S. J. Enhancement of T-cell activation by the CD43 molecule whose expression is defective in Wiskott-Aldrich syndrome. Nature. 1991 Apr 25;350(6320):706–709. doi: 10.1038/350706a0. [DOI] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Piller V., Piller F., Fukuda M. Phosphorylation of the major leukocyte surface sialoglycoprotein, leukosialin, is increased by phorbol 12-myristate 13-acetate. J Biol Chem. 1989 Nov 5;264(31):18824–18831. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Zimmerman C., Kenney D., Rosen F. S. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987 Jul;70(1):104–109. [PubMed] [Google Scholar]
- Rosenstein Y., Park J. K., Hahn W. C., Rosen F. S., Bierer B. E., Burakoff S. J. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991 Nov 21;354(6350):233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Davis A. E., 3rd, Bruns G. A., Rosen F. S., Carroll M. C., Whitehead A. S. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2819–2823. doi: 10.1073/pnas.86.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L. B., Wong R. C., Remold-O'Donnell E., Vercelli D., Sancho J., Terhorst C., Rosen F., Geha R., Chatila T. Mechanism of mononuclear cell activation by an anti-CD43 (sialophorin) agonistic antibody. J Immunol. 1989 Jun 15;142(12):4194–4200. [PubMed] [Google Scholar]
- Smith S. H., Rigley K. P., Callard R. E. Activation of human B cells through the CD19 surface antigen results in homotypic adhesion by LFA-1-dependent and -independent mechanisms. Immunology. 1991 Jul;73(3):293–297. [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Angelisová P., Kristofová H., Horejsí V. Characterization of a 95 kDa human leucocyte sialoglycoprotein: its identity with CD43, gpL115, leukosialin and sialophorin. Folia Biol (Praha) 1988;34(4):255–265. [PubMed] [Google Scholar]
- Stross W. P., Warnke R. A., Flavell D. J., Flavell S. U., Simmons D., Gatter K. C., Mason D. Y. Molecule detected in formalin fixed tissue by antibodies MT1, DF-T1, and L60 (Leu-22) corresponds to CD43 antigen. J Clin Pathol. 1989 Sep;42(9):953–961. doi: 10.1136/jcp.42.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Galea-Lauri J., Sellars R. A., Boniface C. Identification and tissue distribution of rabbit leucocyte antigens recognized by monoclonal antibodies. Immunology. 1992 Aug;76(4):625–630. [PMC free article] [PubMed] [Google Scholar]
- van Noesel C., Miedema F., Brouwer M., de Rie M. A., Aarden L. A., van Lier R. A. Regulatory properties of LFA-1 alpha and beta chains in human T-lymphocyte activation. Nature. 1988 Jun 30;333(6176):850–852. doi: 10.1038/333850a0. [DOI] [PubMed] [Google Scholar]