Abstract
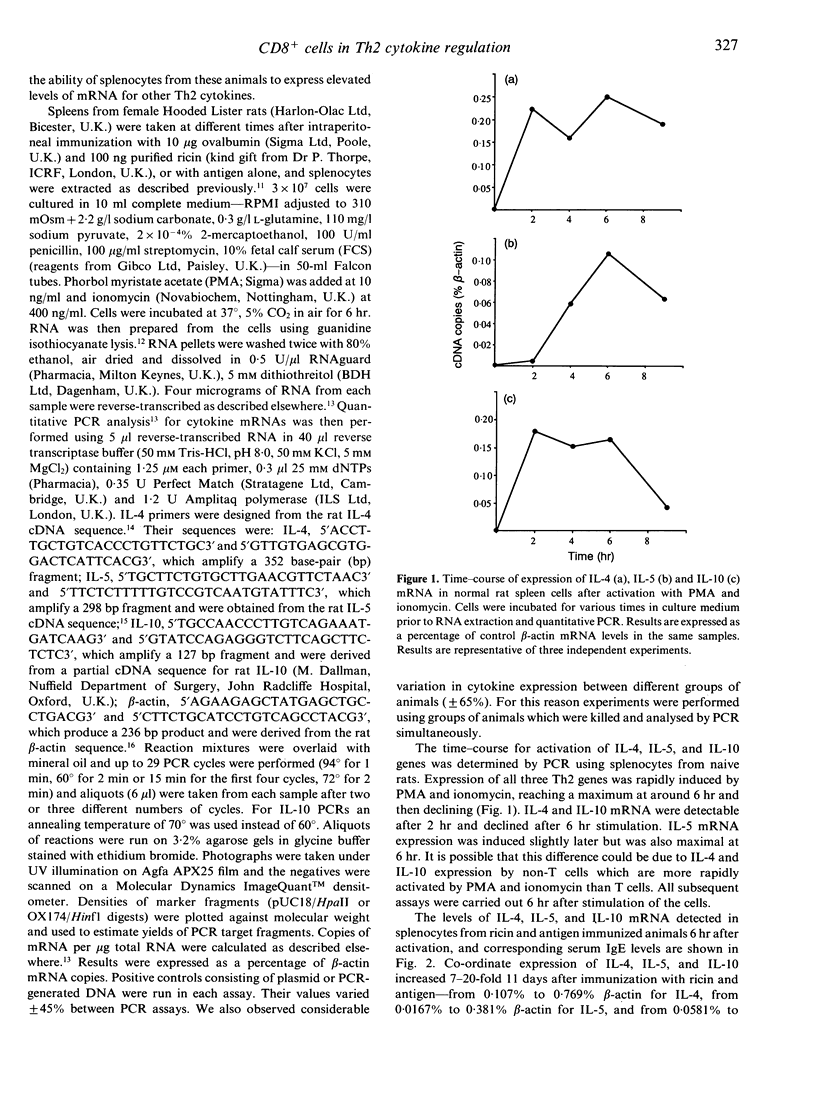
Immunization of rats with soluble antigen (ovalbumin) and the castor bean toxin, ricin, eliminates a subpopulation of CD8+ T cells which suppress IgE responses in vivo. This treatment also reduces the ability of splenic T cells to produce interferon-gamma (IFN-gamma) and enhances their capacity to make interleukin-4 (IL-4). In this report we describe the effect of immunization with ricin and antigen on the expression of mRNA for other T-helper type 2 (Th2) cytokines--IL-5 and IL-10--and their relationship to serum IgE and IL-4 mRNA expression. Splenocytes were taken from rats at different times after immunization with antigen or ricin and antigen and activated in vitro with phorbol myristate acetate (PMA) and ionomycin for 6 hr and total RNA extracted and reverse transcribed. Cytokine gene expression was detected using a quantitative polymerase chain reaction (PCR). Expression of IL-4, IL-5, and IL-10 was increased 7-20-fold 11 days after immunization with ricin and antigen (from 0.107% to 0.769% beta-actin for IL-4, from 0.0167% to 0.381% beta-actin for IL-5, and from 0.0581% to 0.954% beta-actin for IL-10), and preceded maximum serum IgE levels by 4-5 days. There was no increase in IgE or mRNA for these cytokines in rats immunized with antigen alone. The level of IL-4, IL-5, and IL-10 expression declined rapidly after 12 days. Our results suggest that immunization with antigen and ricin preferentially induces a Th2 response, and that CD8+ T cells may play a part in down-regulating the development of Th2 T cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley L. M., Duncan D. D., Tonkonogy S., Swain S. L. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991 Sep 1;174(3):547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Ricci M., Romagnani S. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med. 1991 Oct 1;174(4):809–813. doi: 10.1084/jem.174.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. Generation of a long-lived IgE response in high and low responder strains of rat by co-administration of ricin and antigen. Immunology. 1991 Feb;72(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Lee T. H., Kemeny D. M. Ricin enhances IgE responses by inhibiting a subpopulation of early-activated IgE regulatory CD8+ T cells. Immunology. 1993 Feb;78(2):226–236. [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Barclay A. N., Mason D. W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991 May;21(5):1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkerson J. M., Isakson P. C. Interleukin 5 (IL-5) provides a signal that is required in addition to IL-4 for isotype switching to immunoglobulin (Ig) G1 and IgE. J Exp Med. 1992 Apr 1;175(4):973–982. doi: 10.1084/jem.175.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Lee T. H. Expression of interleukin-5 and granulocyte-macrophage colony-stimulating factor in human peripheral blood mononuclear cells after activation with phorbol myristate acetate. Immunology. 1992 Jan;75(1):196–201. [PMC free article] [PubMed] [Google Scholar]
- Svetić A., Finkelman F. D., Jian Y. C., Dieffenbach C. W., Scott D. E., McCarthy K. F., Steinberg A. D., Gause W. C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991 Oct 1;147(7):2391–2397. [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]


