Abstract
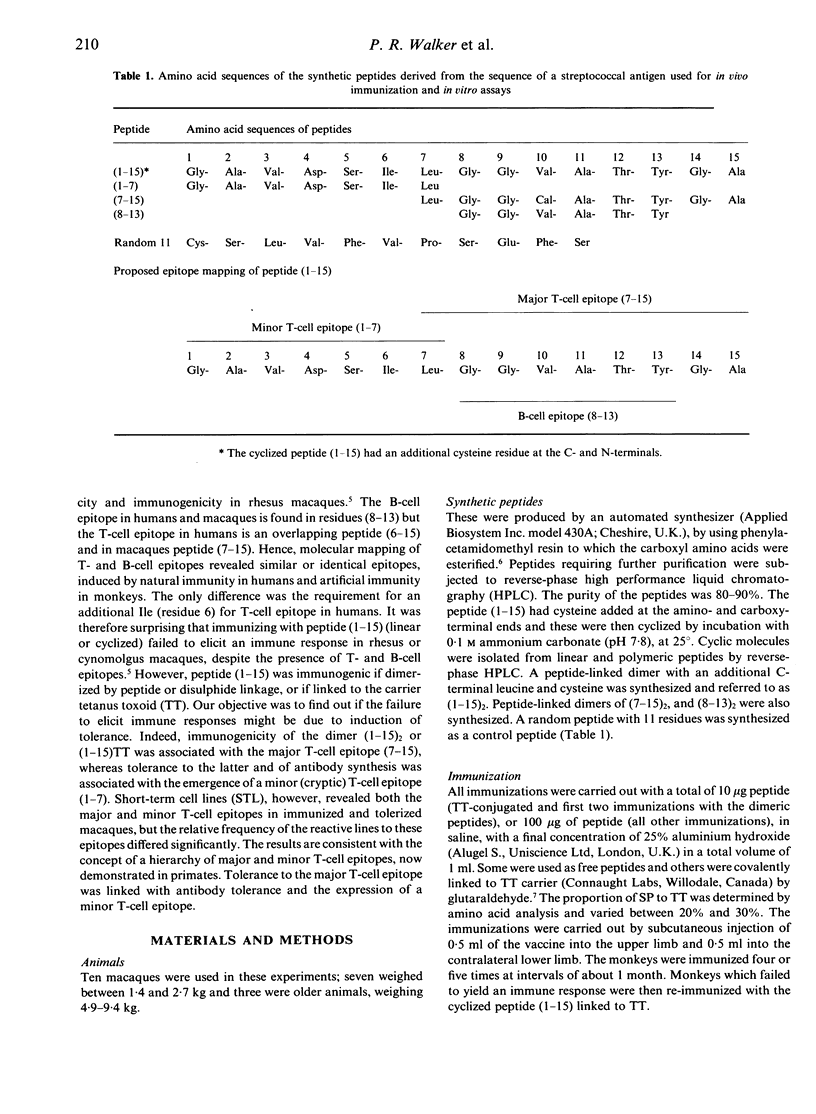
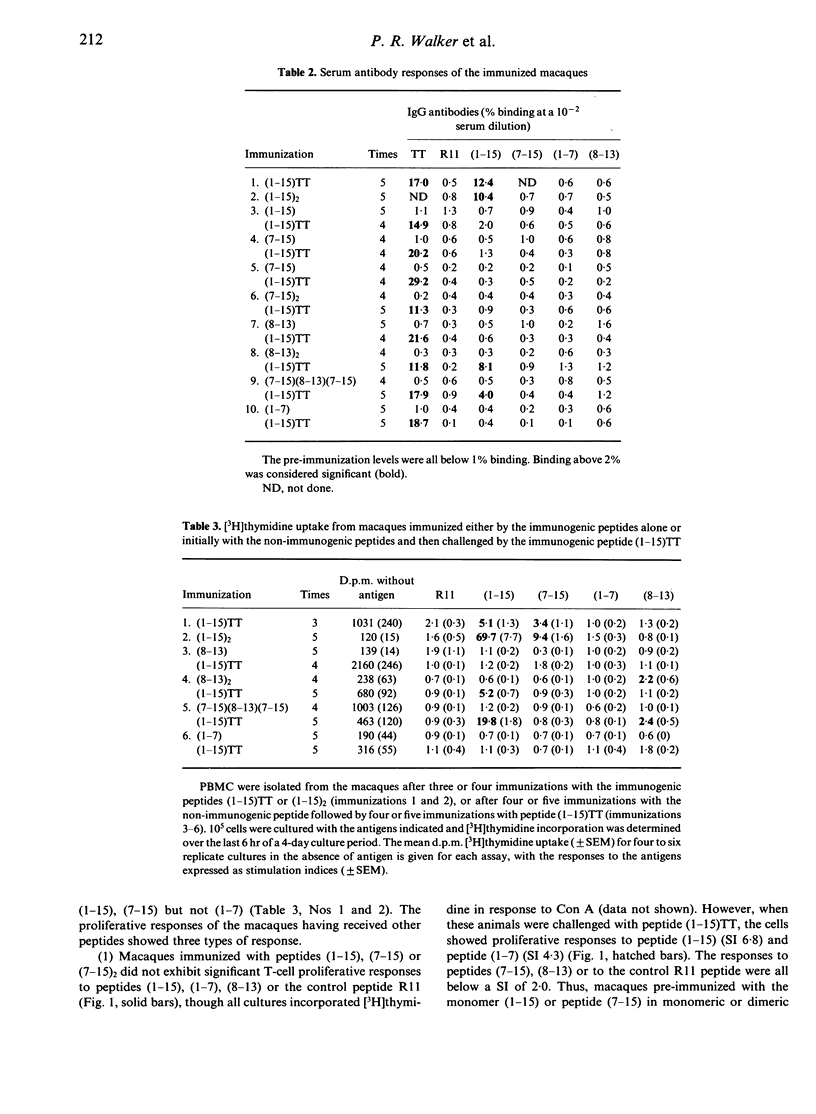
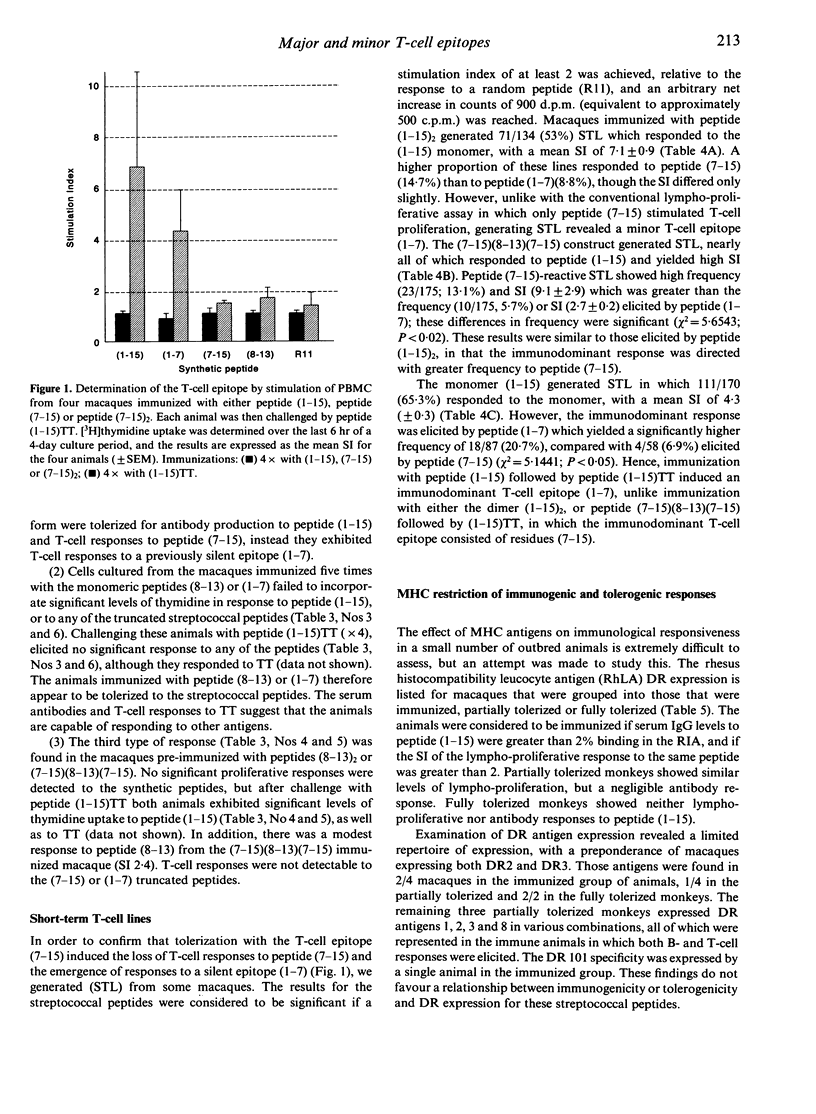
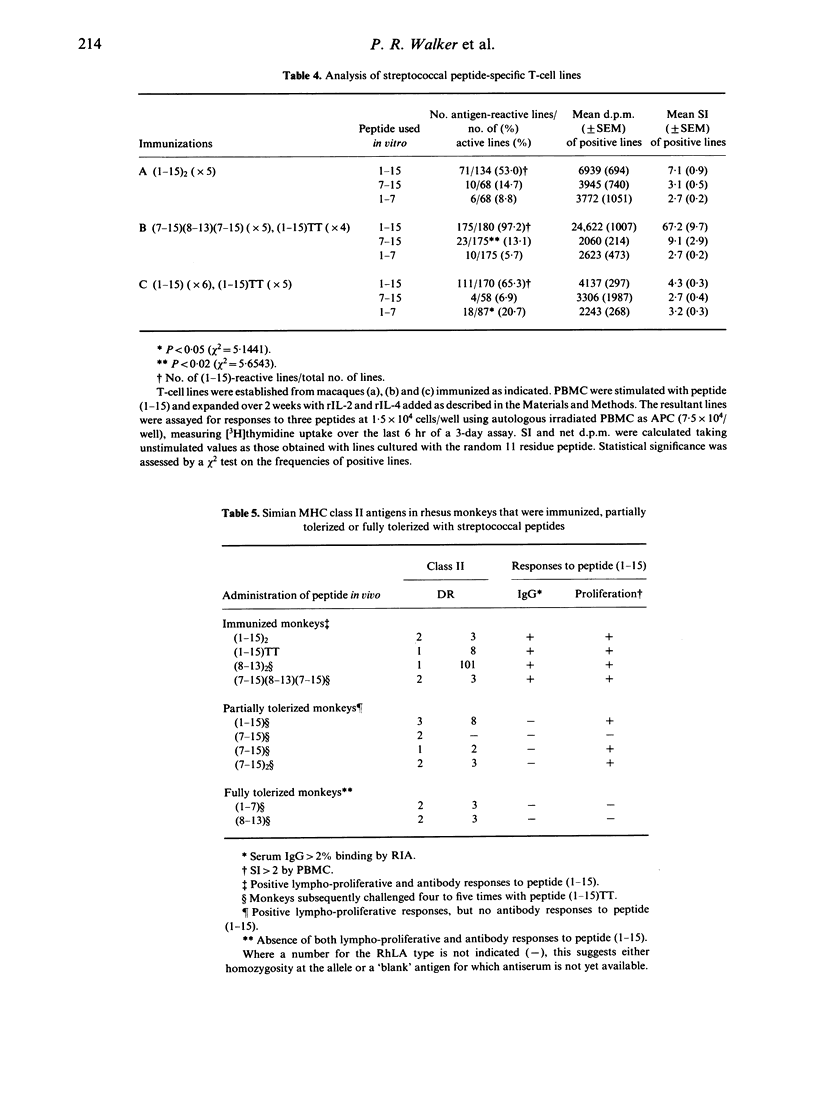
The immunogenicity of synthetic peptides of in vitro mapped T- and B-cell epitopes from a Streptococcus mutans cell-surface antigen were investigated in non-human primates. Peptide (1-15) contains T-cell (7-15) and B-cell (8-13) epitopes, but is only immunogenic if dimerized (1-15)2 or linked to the carrier tetanus toxoid (1-15)TT. Monomers and dimers of T- and B-cell epitopes were prepared and used to immunize macaques. Immunogenicity was assayed in lymphocytes by the uptake of [3H]thymidine and serum antibodies by a solid-phase radioimmunoassay. Macaques immunized with the dimerized (1-15)2 or carrier-linked peptide (1-15)TT exhibited in vitro T-cell proliferative responses to peptides (1-15) and (7-15). T cells from animals immunized with peptides (1-15), (7-15) or (7-15)2 failed to elicit an immune response. In order to establish if these non-immunogenic peptides might induce tolerance, the same macaques were challenged with the immunogenic peptide (1-15)TT. The results suggest that T-cell responses to peptide (1-15) were reestablished, but instead of responding to peptide (7-15) they were stimulated by a hitherto silent epitope (1-7). Tolerance to the major T-cell epitope (7-15) and the expression of a minor (silent) T-cell epitope (1-7) was associated with B-cell tolerance, suggesting that T-cell help for antibodies resides in the major T-cell epitope (7-15). However, short-term T-cell lines revealed T-cell responses to peptides (1-7) and (7-15) in both tolerized and immunized macaques, but the relative frequency of the minor epitope (1-7)-reactive lines was significantly higher in tolerized animals, whilst that for the major epitope (7-15) was higher in immunized animals. These findings suggest that the silent epitope (1-7) is really cryptic, in that it can be detected if the cell lines are first expanded in vitro with the whole peptide (1-15) and then stimulated with the truncated peptides (1-7) or (7-15). The results are consistent with the concept of a hierarchy of major and minor T-cell epitopes, now demonstrated in non-human primates, in which tolerance to the major T-cell epitope is associated with tolerance to antibody formation and the emergence of a minor T-cell epitope.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Nagy Z. A. Peptide competition for antigen presentation. Immunol Today. 1990 Jan;11(1):21–24. doi: 10.1016/0167-5699(90)90006-u. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Childerstone A., Altmann D., Haron J. A., Wilkinson D., Trowsdale J., Lehner T. An analysis of synthetic peptide restriction by HLA-DR alleles in T cells from human subjects, naturally sensitized by Streptococcus mutans. J Immunol. 1991 Mar 1;146(5):1463–1469. [PubMed] [Google Scholar]
- Childerstone A., Haron J., Lehner T. The reactivity of naturally sensitized human CD4 cells and IgG antibodies to synthetic peptides derived from the amino terminal sequences of a 3800 MW Streptococcus mutans antigen. Immunology. 1990 Feb;69(2):177–183. [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Bittle J. L., Houghten R. A., Lerner R. A., Brown F. Immune response to uncoupled peptides of foot-and-mouth disease virus. Immunology. 1987 May;61(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Brown F., McDermed J., Lu Y. A., Tam J. P. Immunological evaluation of the multiple antigen peptide (MAP) system using the major immunogenic site of foot-and-mouth disease virus. Immunology. 1991 Jul;73(3):249–254. [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Hale P. M., Cease K. B., Houghten R. A., Ouyang C., Putney S., Javaherian K., Margalit H., Cornette J. L., Spouge J. L., DeLisi C. T cell multideterminant regions in the human immunodeficiency virus envelope: toward overcoming the problem of major histocompatibility complex restriction. Int Immunol. 1989;1(4):409–415. doi: 10.1093/intimm/1.4.409. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Hollosi M., Dietzschold B., Hudecz F., Fasman G. D. The T cell response to the glycoprotein D of the herpes simplex virus: the significance of antigen conformation. J Immunol. 1985 Aug;135(2):1385–1390. [PubMed] [Google Scholar]
- Lehner T., Walker P., Bergmeier L. A., Haron J. A. Immunogenicity of synthetic peptides derived from the sequences of a Streptococcus mutans cell surface antigen in nonhuman primates. J Immunol. 1989 Oct 15;143(8):2699–2705. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Partidos C. D., Steward M. W. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990 Sep;71(Pt 9):2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- Ria F., Chan B. M., Scherer M. T., Smith J. A., Gefter M. L. Immunological activity of covalently linked T-cell epitopes. Nature. 1990 Jan 25;343(6256):381–383. doi: 10.1038/343381a0. [DOI] [PubMed] [Google Scholar]
- Sercarz E., Krzych U. The distinctive specificity of antigen-specific suppressor T cells. Immunol Today. 1991 Apr;12(4):111–118. doi: 10.1016/0167-5699(91)90094-A. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Smith R., Lehner T. A radioimmunoassay for serum and gingival crevicular fluid antibodies to a purified protein of Streptococcus mutans. Clin Exp Immunol. 1981 Feb;43(2):417–424. [PMC free article] [PubMed] [Google Scholar]


