Abstract
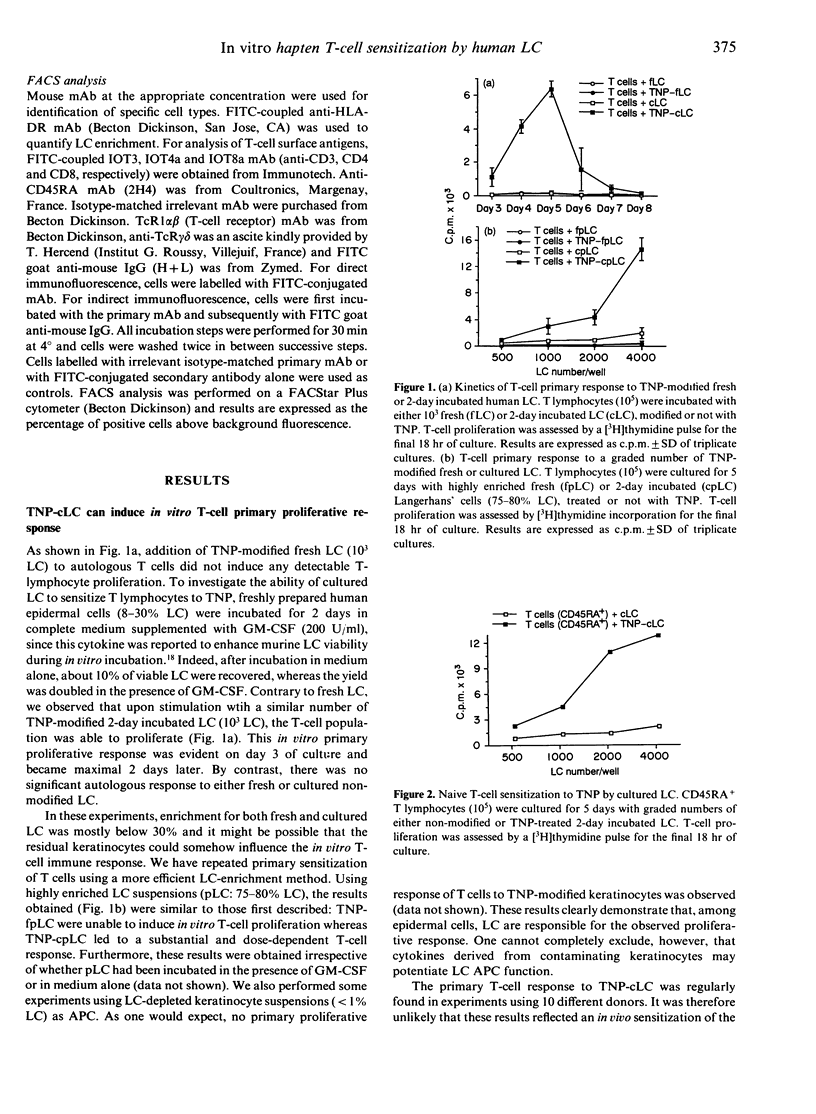
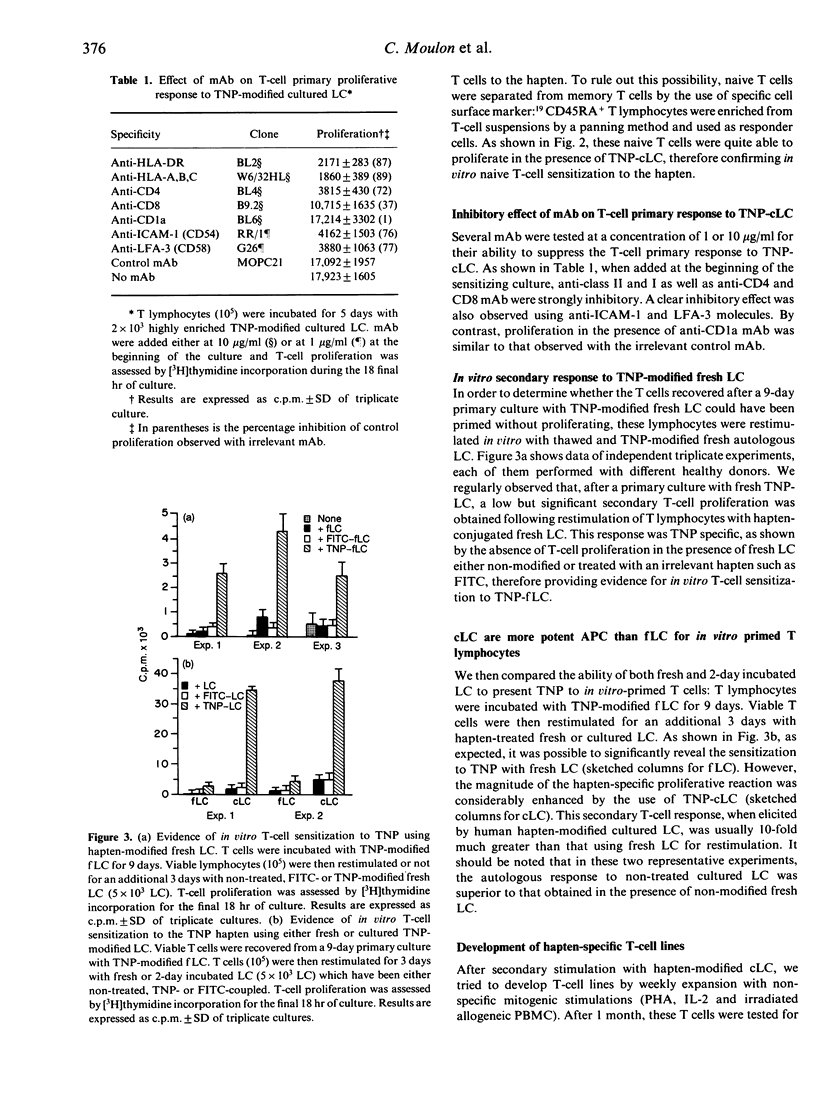
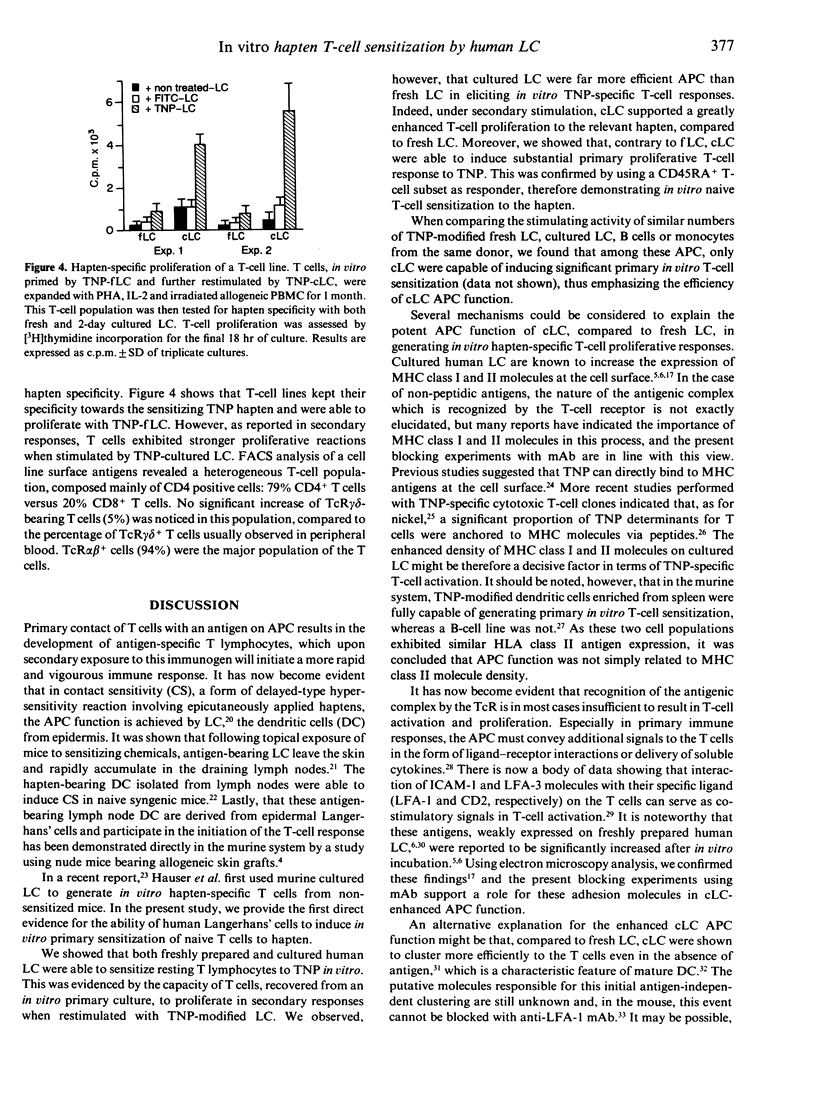
We examined the capacity of human Langerhans's cells (LC) to sensitize autologous T cells to the trinitrophenyl hapten (TNP) in vitro. Two-day cultured Langerhans' cells, but not freshly prepared Langerhans' cells, can induce in vitro primary proliferative reactions to the TNP hapten. Using a CD45RA+ naive T-cell subset, similar results were found, therefore making the possibility of a previous in vivo T-cell contact with the hapten unlikely. The primary in vitro response was strongly inhibited by monoclonal antibodies to major histocompatibility complex (MHC) class I and II, CD4 antigens and ICAM-1 and LFA-3 adhesion molecules. Furthermore, we found that fresh LC can prime T cells to TNP, as revealed by a significant secondary T-cell proliferation after restimulation of the recovered T lymphocytes by fresh hapten-modified autologous LC. Nevertheless, the ability of these fresh LC to stimulate in vitro secondary hapten-specific T-cell proliferation was very limited in comparison with that of 2-day incubated Langerhans' cells. After secondary stimulation with TNP-cultured LC, sensitized T cells could be non-specifically expanded without losing hapten specificity. The TNP-specific T-cell lines were mostly of the CD4+ phenotype. The present findings extend previous studies in the mouse, showing that culture LC are potent antigen-presenting cells (APC) in primary hapten-dependent proliferation assays. Furthermore, this in vitro priming assay, using cultured human Langerhans' cells as APC, might be useful to analyse the early steps of T-cell sensitization and subsequently to develop in vitro predictive tests allowing detection of sensitizing compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Katz S. I. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990 Nov 1;145(9):2791–2796. [PubMed] [Google Scholar]
- Akbar A. N., Salmon M., Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991 Jun;12(6):184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- Charmot D., Mawas C. The in vitro cellular response of human lymphocytes to trinitrophenylated autologous cells: HLA-D restriction of proliferation but apparent absence of HLA restriction of cytolysis. Eur J Immunol. 1979 Sep;9(9):723–730. doi: 10.1002/eji.1830090911. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990 Nov;71(3):404–410. [PMC free article] [PubMed] [Google Scholar]
- Forman J., Vitetta E. S., Hart D. A., Klein J. Relationship between trinitrophenyl and H-2 antigens on trinitrophenyl-modified spleen cells. I. H-2 antigens on cells treated with trinitrobenzene sulfonic acid are derivatized. J Immunol. 1977 Mar;118(3):797–802. [PubMed] [Google Scholar]
- Geppert T. D., Davis L. S., Gur H., Wacholtz M. C., Lipsky P. E. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990 Oct;117:5–66. doi: 10.1111/j.1600-065x.1990.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Gocinski B. L., Tigelaar R. E. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990 Jun 1;144(11):4121–4128. [PubMed] [Google Scholar]
- Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J Invest Dermatol. 1990 Oct;95(4):436–440. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- Hauser C., Katz S. I. Generation and characterization of T-helper cells by primary in vitro sensitization using Langerhans cells. Immunol Rev. 1990 Oct;117:67–84. doi: 10.1111/j.1600-065x.1990.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Hauser C., Yokoyama W. M., Katz S. I. Characterization of primary T helper cell activation and T helper cell lines stimulated by hapten-modified, cultured Langerhans cells. J Invest Dermatol. 1989 Nov;93(5):649–655. doi: 10.1111/1523-1747.ep12319814. [DOI] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Monoclonal antibodies to LFA-1 and to CD4 inhibit the mixed leukocyte reaction after the antigen-dependent clustering of dendritic cells and T lymphocytes. J Exp Med. 1987 May 1;165(5):1403–1417. doi: 10.1084/jem.165.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke M. L., Munn C. G., Jeevan A., Tang J. M., Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990 Nov 1;145(9):2833–2838. [PubMed] [Google Scholar]
- Macatonia S. E., Edwards A. J., Knight S. C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986 Dec;59(4):509–514. [PMC free article] [PubMed] [Google Scholar]
- Ortmann B., Martin S., von Bonin A., Schiltz E., Hoschützky H., Weltzien H. U. Synthetic peptides anchor T cell-specific TNP epitopes to MHC antigens. J Immunol. 1992 Mar 1;148(5):1445–1450. [PubMed] [Google Scholar]
- Picut C. A., Lee C. S., Dougherty E. P., Anderson K. L., Lewis R. M. Immunostimulatory capabilities of highly enriched Langerhans cells in vitro. J Invest Dermatol. 1988 Feb;90(2):201–206. doi: 10.1111/1523-1747.ep12462221. [DOI] [PubMed] [Google Scholar]
- Puré E., Inaba K., Crowley M. T., Tardelli L., Witmer-Pack M. D., Ruberti G., Fathman G., Steinman R. M. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990 Nov 1;172(5):1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péguet-Navarro J., Dalbiez-Gauthier C., Dezutter-Dambuyant C., Schmitt D. Dissection of human Langerhans cells' allostimulatory function: the need for an activation step for full development of accessory function. Eur J Immunol. 1993 Feb;23(2):376–382. doi: 10.1002/eji.1830230212. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Labhardt A. M., Sinigaglia F. Selective interaction of Ni with an MHC-bound peptide. EMBO J. 1991 Jun;10(6):1303–1306. doi: 10.1002/j.1460-2075.1991.tb07648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Lenz A., Glassel H., Stössel H., Stanzl U., Majdic O., Fritsch P., Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989 Nov;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin M. F., Rich R. R. Human immune responses to hapten-conjugated cells. I. Primary and secondary proliferative responses in vitro. J Exp Med. 1978 Jun 1;147(6):1671–1683. doi: 10.1084/jem.147.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Shimada S., Caughman S. W., Sharrow S. O., Stephany D., Katz S. I. Enhanced antigen-presenting capacity of cultured Langerhans' cells is associated with markedly increased expression of Ia antigen. J Immunol. 1987 Oct 15;139(8):2551–2555. [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Gigli I., Baer R. L., Thorbecke G. J. Langerhans cells: role in contact hypersensitivity and relationship to lymphoid dendritic cells and to macrophages. Immunol Rev. 1980;53:203–232. doi: 10.1111/j.1600-065x.1980.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Grammer S. F. In vitro evidence that Langerhans cells can adopt two functionally distinct forms capable of antigen presentation to T lymphocytes. J Immunol. 1989 Dec 15;143(12):3925–3933. [PubMed] [Google Scholar]
- Sullivan S., Bergstresser P. R., Tigelaar R. E., Streilein J. W. Induction and regulation of contact hypersensitivity by resident, bone marrow-derived, dendritic epidermal cells: Langerhans cells and Thy-1+ epidermal cells. J Immunol. 1986 Oct 15;137(8):2460–2467. [PubMed] [Google Scholar]
- Tang A., Udey M. C. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation. Ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J Immunol. 1991 May 15;146(10):3347–3355. [PubMed] [Google Scholar]
- Teunissen M. B., Wormmeester J., Krieg S. R., Peters P. J., Vogels I. M., Kapsenberg M. L., Bos J. D. Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol. 1990 Feb;94(2):166–173. doi: 10.1111/1523-1747.ep12874439. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Bergstresser P. R., Streilein J. W. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980 Jan;124(1):445–453. [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]


