Abstract
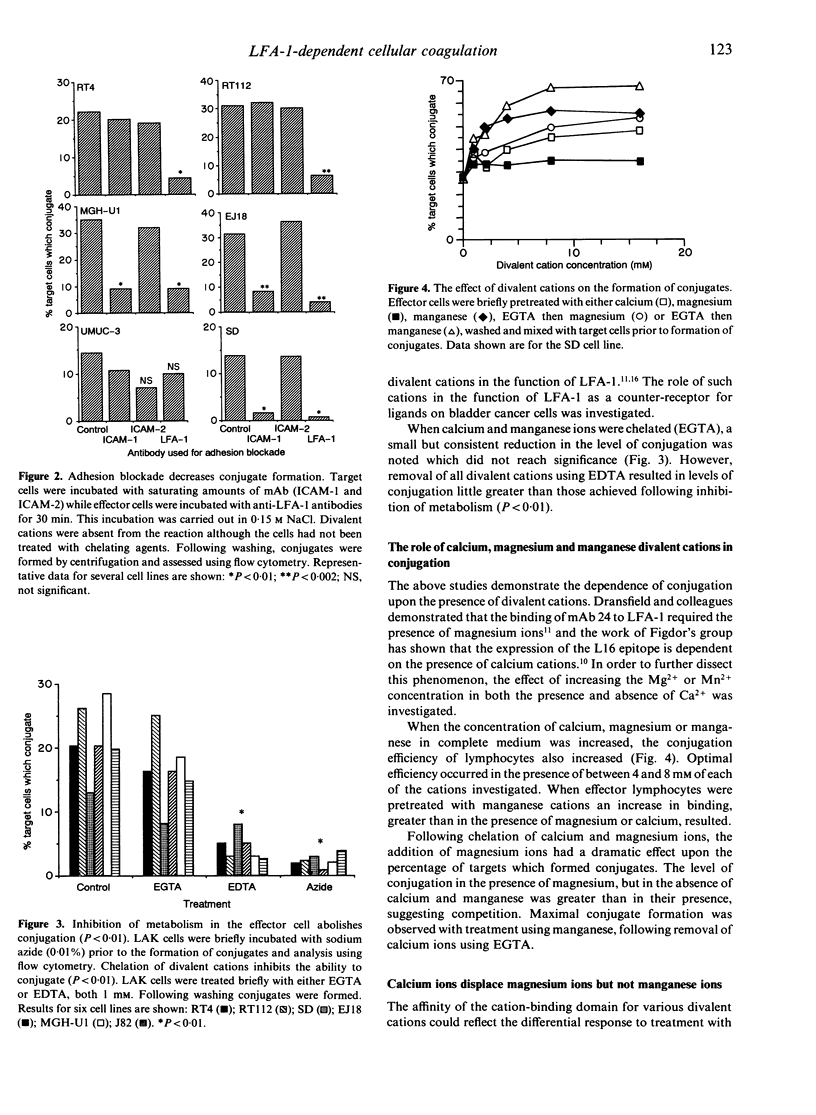
The control of integrin activation is fundamental to an understanding of the integrin-dependent cellular adhesion thought to be important for a plethora of basic cellular functions. Using a cell-cell conjugation assay the role of divalent cations in leucocyte function-associated antigen-1 (LFA-1)-dependent cellular adhesion was further investigated. The conjugation of interleukin-2 (IL-2)-activated lymphocytes to tumour cells was found to be energy dependent and required the presence of various divalent cations, removal of which decreased the level of conjugation. Increased concentrations of calcium, magnesium and manganese ions resulted in a corresponding increase in levels of conjugation. This increase in conjugation was LFA-1 dependent. Interestingly, when calcium ions were first removed from LFA-1, treatment of lymphocytes with magnesium and manganese ions gave significantly higher levels of conjugation than in the presence of calcium. Using a simple displacement study, calcium ions were shown to displace magnesium ions resulting in decreased conjugation. However, calcium ions were unable to displace manganese ions for binding to LFA-1. That manganese was exerting its effect via an LFA-1-dependent mechanism was confirmed using monoclonal antibodies to CD11a which negated the increased conjugation frequency due to manganese.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavarec L., Quillet-Mary A., Fradelizi D., Conjeaud H. An improved double fluorescence flow cytometry method for the quantification of killer cell/target cell conjugate formation. J Immunol Methods. 1990 Jul 3;130(2):251–261. doi: 10.1016/0022-1759(90)90055-z. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Dransfield I., Hogg N. Identification of a novel monocyte cell surface molecule involved in the generation of antigen-induced proliferative responses. Eur J Immunol. 1988 Dec;18(12):2067–2071. doi: 10.1002/eji.1830181229. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Buckle A. M., Hogg N. Early events of the immune response mediated by leukocyte integrins. Immunol Rev. 1990 Apr;114:29–44. doi: 10.1111/j.1600-065x.1990.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Barrett J., Hogg N. Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. J Cell Biol. 1992 Mar;116(6):1527–1535. doi: 10.1083/jcb.116.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992 Jan;116(1):219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989 Dec 1;8(12):3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Figdor C. G., van Kooyk Y., Keizer G. D. On the mode of action of LFA-1. Immunol Today. 1990 Aug;11(8):277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. M., Alexandrov A. B., Prescott S., James K., Chisholm G. D. Role of adhesion molecules in lymphokine-activated killer cell killing of bladder cancer cells: further evidence for a third ligand for leucocyte function-associated antigen-1. Immunology. 1992 Jun;76(2):286–291. [PMC free article] [PubMed] [Google Scholar]
- Jackson A. M., Hawkyard S. J., Prescott S., Ritchie A. W., James K., Chisholm G. D. An investigation of factors influencing the in vitro induction of LAK activity against a variety of human bladder cancer cell lines. J Urol. 1992 Jan;147(1):207–211. doi: 10.1016/s0022-5347(17)37198-7. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Visser W., Vliem M., Figdor C. G. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988 Mar 1;140(5):1393–1400. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Martz E. LFA-1 and other accessory molecules functioning in adhesions of T and B lymphocytes. Hum Immunol. 1987 Jan;18(1):3–37. doi: 10.1016/0198-8859(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Kozumbo W. J., Moretta L., Moretta A. Transmembrane signalling via the T11-dependent pathway of human T cell activation. Evidence for the involvement of 1,2-diacylglycerol and inositol phosphates. Eur J Immunol. 1987 Jan;17(1):55–60. doi: 10.1002/eji.1830170110. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman J. B., Pasqualini R., Takada Y., Hemler M. E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993 Apr 25;268(12):8651–8657. [PubMed] [Google Scholar]
- de Fougerolles A. R., Springer T. A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992 Jan 1;175(1):185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y., Weder P., Hogervorst F., Verhoeven A. J., van Seventer G., te Velde A. A., Borst J., Keizer G. D., Figdor C. G. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991 Jan;112(2):345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]


