Abstract
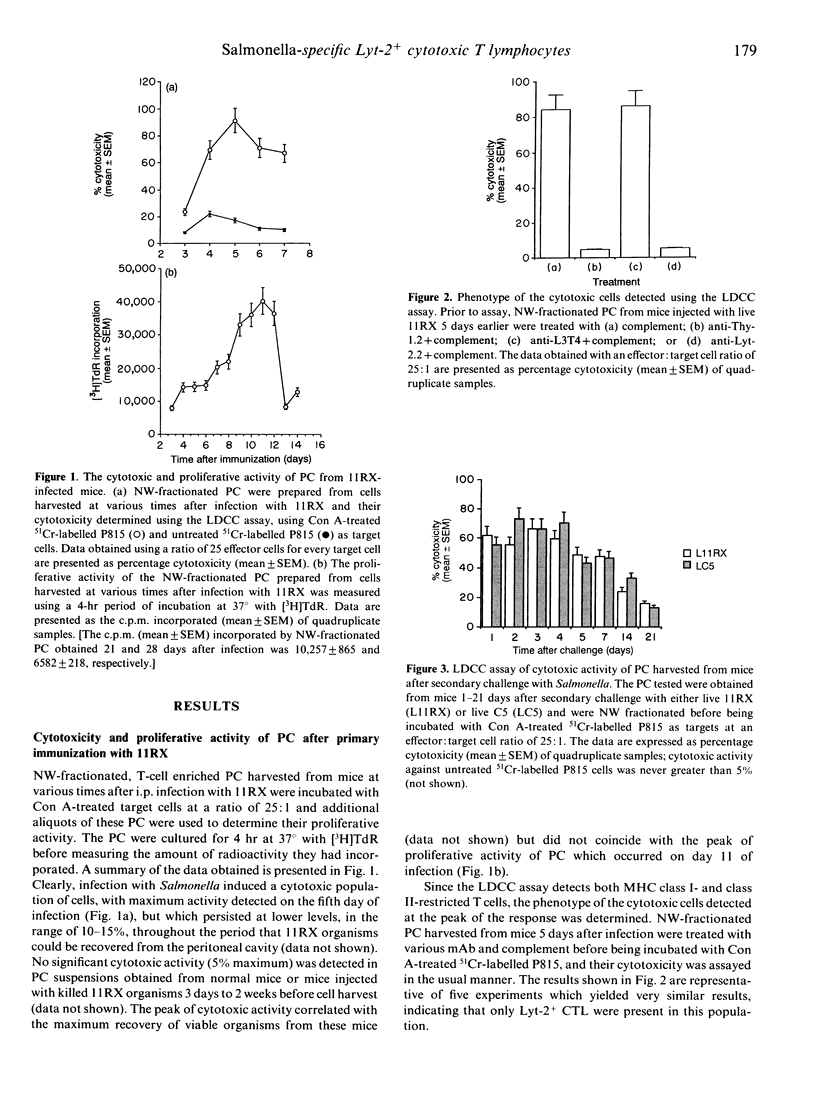
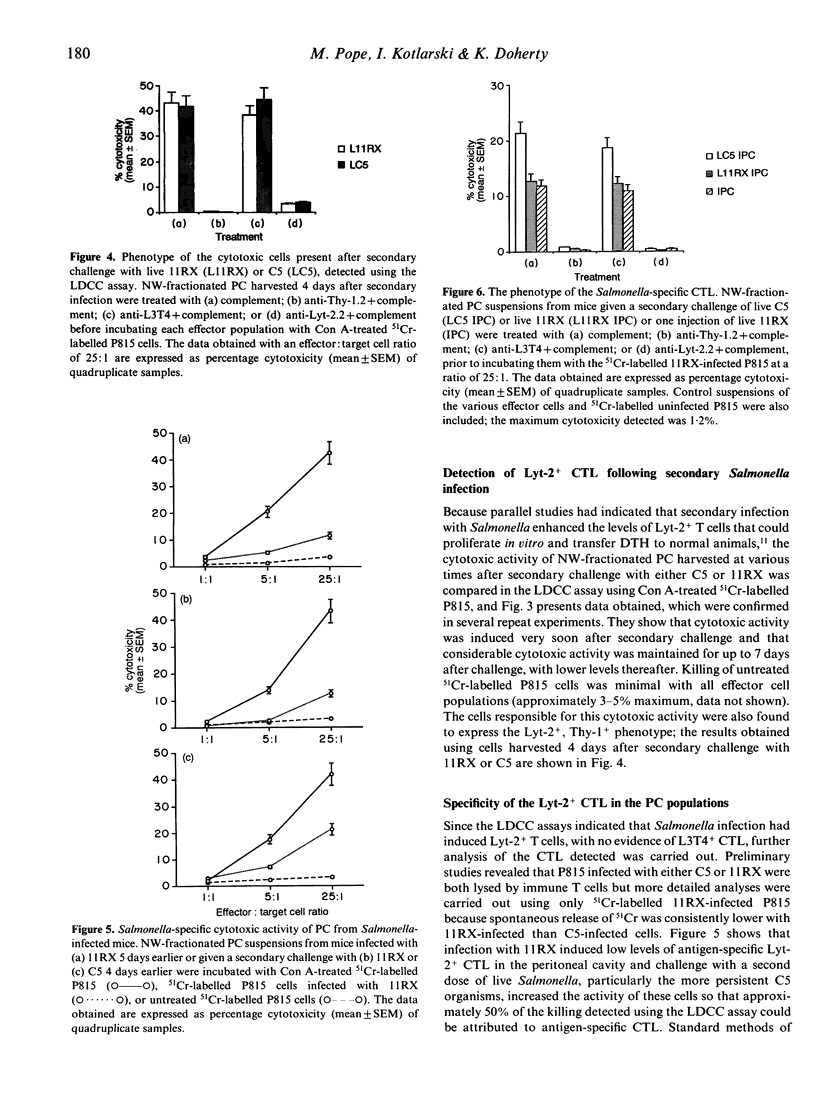
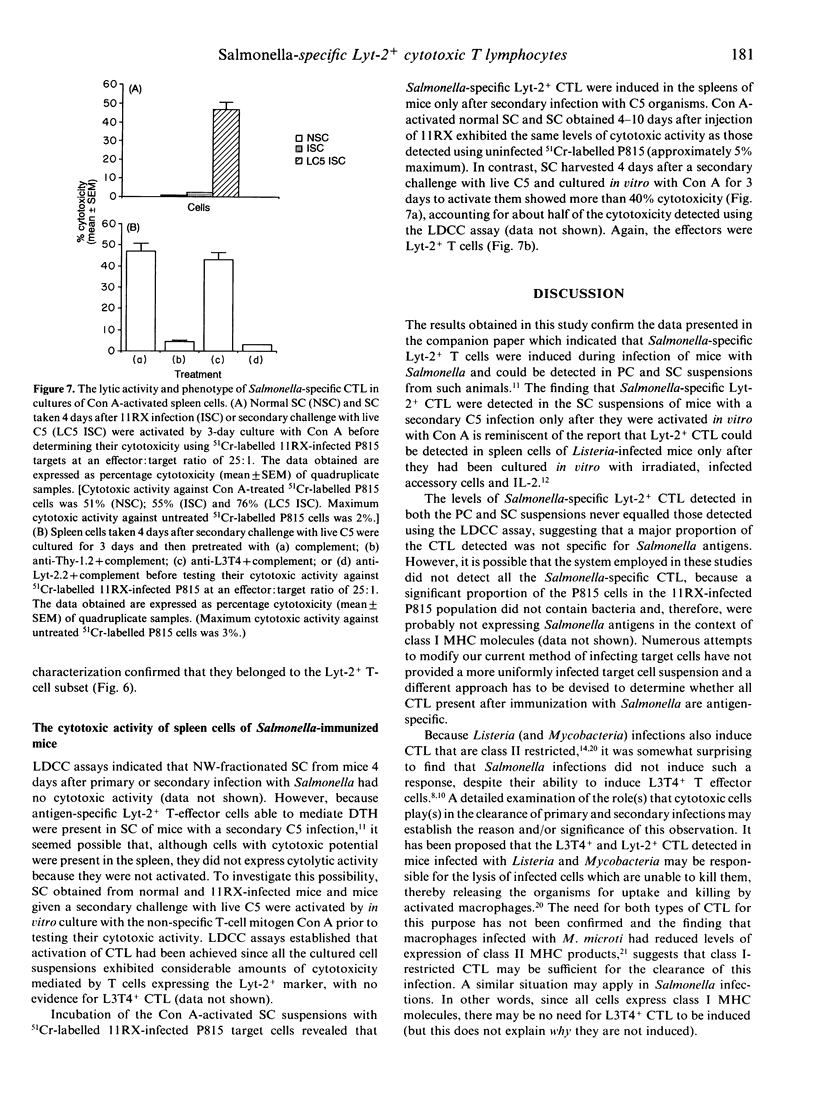
Investigations of the cytotoxic activity of T cells induced following one or two intraperitoneal doses of live Salmonella revealed that cytotoxicity was restricted to the Lyt-2+ T-cell subset and was enhanced following secondary infection with Salmonella. Initial studies using the lectin-dependent cellular cytotoxicity (LDCC) assay detected Lyt-2+ cytotoxic T cells in peritoneal cell suspensions of S. enteritidis 11RX (11RX)-infected mice, with the peak of activity occurring 5 days after infection. This did not correlate with the proliferative activity of these cells, which peaked 10-12 days after infection. Secondary challenge with 11RX or S. typhimurium C5 (C5) induced a rapid increase in the cytotoxic activity of Lyt-2+ peritoneal T cells and was detected even 21 days later. The antigen specificity of some of these cells was confirmed in cytotoxicity assays using P815 tumour cells infected with 11RX organisms as targets. No cytotoxic activity was detected in the spleen cell suspensions of infected (and normal) mice unless the cells were first activated by in vitro culture with concanavalin A (Con A). Both types of activated spleen cells showed LDCC but Salmonella-specific cytotoxic Lyt-2+ T cells were detected only in spleen cell (SC) cultures prepared from mice challenged with a second dose of Salmonella.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge S. R., Kotlarski I. In vitro lymphokine release by lymphocytes from mice infected with Salmonella. Aust J Exp Biol Med Sci. 1985 Oct;63(Pt 5):489–502. doi: 10.1038/icb.1985.53. [DOI] [PubMed] [Google Scholar]
- Bevan M. J., Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol. 1975 Feb;114(2 Pt 1):559–565. [PubMed] [Google Scholar]
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Bonavida B., Bradley T. P. Studies on the induction and expression of T cell-mediated immunity. V. Lectin-induced nonspecific cell-mediated cytotoxicity by alloimmune lymphocytes. Transplantation. 1976 Feb;21(2):94–102. doi: 10.1097/00007890-197602000-00002. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. P., Bonavida B. Studies on the induction and expression of T cell-mediated immunity. VII. Inactivation of autologous cytotoxic T lymphocytes when used as both effectors and targets in a lectin-dependent cellular cytotoxic reaction. Transplantation. 1978 Oct;26(4):212–217. [PubMed] [Google Scholar]
- Davies R., Kotlarski I. The role of thymus-derived cells in immunity to salmonella infection. Aust J Exp Biol Med Sci. 1976 Jun;54(3):221–236. doi: 10.1038/icb.1976.23. [DOI] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Sims M., Feldmann M. Regulation of macrophage accessory cell activity by mycobacteria. II. In vitro inhibition of Ia expression by Mycobacterium microti. Clin Exp Immunol. 1986 Apr;64(1):28–34. [PMC free article] [PubMed] [Google Scholar]
- Kotlarski I., Pope M., Doherty K., Attridge S. R. The in vitro proliferative response of lymphoid cells of mice infected with Salmonella enteritidis 11RX. Immunol Cell Biol. 1989 Feb;67(Pt 1):19–29. doi: 10.1038/icb.1989.3. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Editorial: Delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Mar;111(3):243–246. doi: 10.1164/arrd.1975.111.3.243. [DOI] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol. 1990 Aug 15;145(4):1265–1269. [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Pope M., Kotlarski I. Detection of Salmonella-specific L3T4+ and Lyt-2+ T cells which can proliferate in vitro and mediate delayed-type hypersensitivity reactivity. Immunology. 1994 Feb;81(2):183–191. [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Gao X. M., Hughes-Jenkins C. M., Lipscombe M., O'Callaghan D., Dougan G., Liew F. Y. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. III. Delivery of recombinant nucleoprotein to the immune system using attenuated Salmonella typhimurium as a live carrier. Immunology. 1990 Aug;70(4):540–546. [PMC free article] [PubMed] [Google Scholar]


