Abstract
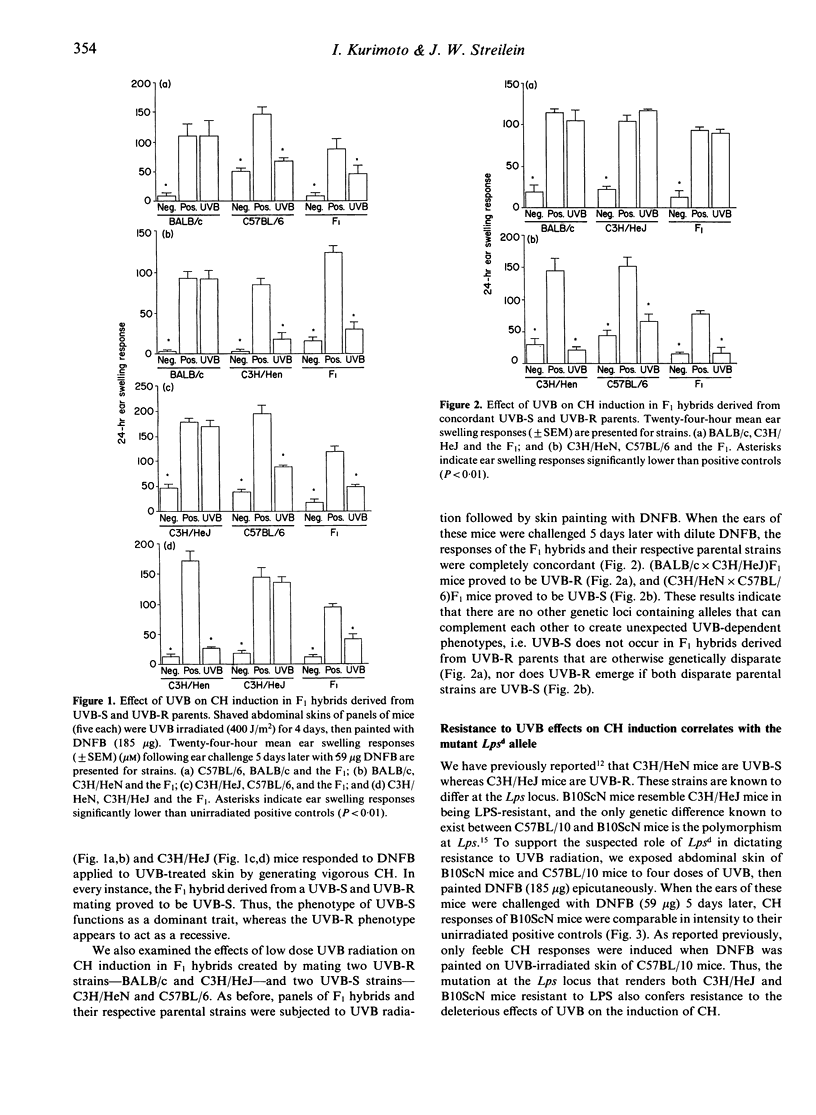
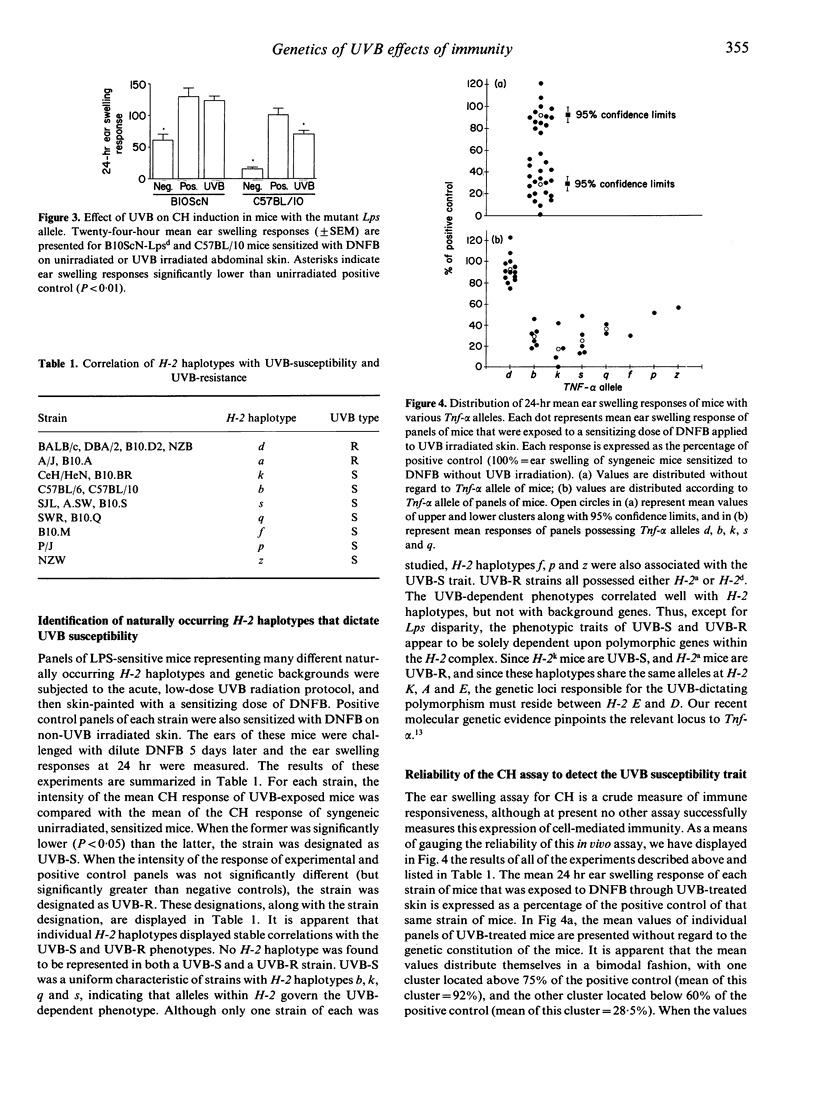
Ultraviolet-B (UVB) light has proven to be deleterious to the skin immune system in mice, and one major consequence is impairment of the induction of contact hypersensitivity (CH) to haptens applied to UVB-exposed skin. It has been shown recently that the damaging effects of UVB on CH are mediated primarily by tumour necrosis factor-alpha (TNF-alpha). Moreover, not all strains of mice are equally susceptible to the deleterious effects of UVB. Mice that develop CH when hapten is applied to UVB-exposed skin are termed UVB-resistant (UVB-R), whereas mice that fail to acquire CH under these circumstances are termed UVB-susceptible (UVB-S). In the present experiments, we have characterized the UVB-susceptibility of numerous, genetically disparate inbred strains of mice by applying dinitrofluorobenzene (DNFB) epicutaneously to normal and to UVB-exposed body wall skin. The results indicate that the intensities of CH responses of these different strains were distributed in a bimodal fashion, with means at 92% and 28.5% of positive control responses. Among the strains with CH values distributed around the higher mean (i.e. UVB-R mice), the intensity of CH responses after UVB irradiation was uniformly greater than 75% of the intensity found among their positive controls. By contrast, among the strains with CH values distributed around the lower mean (i.e. UVB-S mice), the intensity of CH responses after UVB exposure was uniformly less than 60% of the intensity displayed by their positive controls. The phenotypic traits of UVB-S and UVB-R appear, therefore, to be genetically determined. To that end, we provide in this report additional evidence that UVB-S is a polygenically determined trait that is dictated by polymorphisms at a locus within H-2, and at the Lps locus. Resistance to UVB radiation is a recessive trait, and requires homozygosity of resistance alleles at one or both of the two participating loci, whereas UVB-S acts as a dominant trait. Among H-2 congenic strains of mice that are lipopolysaccharide (LPS)-sensitive (Lpsn), UVB radiation impaired the induction of CH to DNFB in all mice except those of the H-2d and H-2a haplotypes. Thus, UVB-susceptibility is dictated by alleles at two, independent genetic loci that can influence transcriptional and translational activity of the Tnf-alpha gene. The potential biological and medical meaning of regulatory polymorphisms governing TNF-alpha production in the skin may be revealed by the recent demonstration that UVB-susceptibility and UVB-resistance are phenotypic traits in humans.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer J., Ganter U., Geiger T., Jacobshagen U., Hirano T., Matsuda T., Kishimoto T., Andus T., Acs G., Gerok W. Regulation of interleukin-6 expression in cultured human blood monocytes and monocyte-derived macrophages. Blood. 1988 Oct;72(4):1134–1140. [PubMed] [Google Scholar]
- Bergstresser P. R., Fletcher C. R., Streilein J. W. Surface densities of Langerhans cells in relation to rodent epidermal sites with special immunologic properties. J Invest Dermatol. 1980 Feb;74(2):77–80. doi: 10.1111/1523-1747.ep12519909. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- De Fabo E. C., Noonan F. P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983 Jul 1;158(1):84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkins Y., Fidler I. J., Kripke M. L. Exposure of mice to UV-B radiation suppresses delayed hypersensitivity to Candida albicans. Photochem Photobiol. 1989 May;49(5):615–619. doi: 10.1111/j.1751-1097.1989.tb08432.x. [DOI] [PubMed] [Google Scholar]
- Everett M. A., Yeargers E., Sayre R. M., Olson R. L. Penetration of epidermis by ultraviolet rays. Photochem Photobiol. 1966 Jul;5(7):533–542. doi: 10.1111/j.1751-1097.1966.tb09843.x. [DOI] [PubMed] [Google Scholar]
- Freund Y. R., Sgarlato G., Jacob C. O., Suzuki Y., Remington J. S. Polymorphisms in the tumor necrosis factor alpha (TNF-alpha) gene correlate with murine resistance to development of toxoplasmic encephalitis and with levels of TNF-alpha mRNA in infected brain tissue. J Exp Med. 1992 Mar 1;175(3):683–688. doi: 10.1084/jem.175.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring L., Baltz M., Pepys M. B., Daynes R. Effect of ultraviolet radiation on production of epidermal cell thymocyte-activating factor/interleukin 1 in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1198–1202. doi: 10.1073/pnas.81.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini M. S. Suppression of pathogenesis in cutaneous leishmaniasis by UV irradiation. Infect Immun. 1986 Mar;51(3):838–843. doi: 10.1128/iai.51.3.838-843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. E., Findley G. B., Jr, Klenk K. F., Wilson W. M., Mo T. The ultraviolet dose dependence of non-melanoma skin cancer incidence. Photochem Photobiol. 1976 Oct;24(4):353–362. doi: 10.1111/j.1751-1097.1976.tb06836.x. [DOI] [PubMed] [Google Scholar]
- Han J., Brown T., Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990 Feb 1;171(2):465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D. J., Lee J. C., Young P. R. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J Biol Chem. 1988 Jun 15;263(17):8473–8479. [PubMed] [Google Scholar]
- Howie S., Norval M., Maingay J. Exposure to low-dose ultraviolet radiation suppresses delayed-type hypersensitivity to herpes simplex virus in mice. J Invest Dermatol. 1986 Feb;86(2):125–128. doi: 10.1111/1523-1747.ep12284128. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Fronek Z., Lewis G. D., Koo M., Hansen J. A., McDevitt H. O. Heritable major histocompatibility complex class II-associated differences in production of tumor necrosis factor alpha: relevance to genetic predisposition to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1233–1237. doi: 10.1073/pnas.87.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Jeevan A., Kripke M. L. Effect of a single exposure to ultraviolet radiation on Mycobacterium bovis bacillus Calmette-Guerin infection in mice. J Immunol. 1989 Nov 1;143(9):2837–2843. [PubMed] [Google Scholar]
- Jongeneel C. V., Acha-Orbea H., Blankenstein T. A polymorphic microsatellite in the tumor necrosis factor alpha promoter identifies an allele unique to the NZW mouse strain. J Exp Med. 1990 Jun 1;171(6):2141–2146. doi: 10.1084/jem.171.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias N., Baqer A. H. Quantitative assessment of UV-induced pigmentation and erythema. Photodermatol. 1988 Feb;5(1):53–60. [PubMed] [Google Scholar]
- Kurimoto I., Streilein J. W. cis-urocanic acid suppression of contact hypersensitivity induction is mediated via tumor necrosis factor-alpha. J Immunol. 1992 May 15;148(10):3072–3078. [PubMed] [Google Scholar]
- Köck A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J. C., Luger T. A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990 Dec 1;172(6):1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Yang D. M., Severn A., Cox F. E. TNF-alpha reverses the disease-exacerbating effect of subcutaneous immunization against murine cutaneous leishmaniasis. Immunology. 1991 Oct;74(2):304–309. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Parkinson C., Millott S., Severn A., Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- Rae V., Yoshikawa T., Bruins-Slot W., Streilein J. W., Taylor J. R. An ultraviolet B radiation protocol for complete depletion of human epidermal Langerhans cells. J Dermatol Surg Oncol. 1989 Nov;15(11):1199–1202. doi: 10.1111/j.1524-4725.1989.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Richter G., Qin Z. H., Diamantstein T., Blankenstein T. Analysis of restriction fragment length polymorphism in lymphokine genes of normal and autoimmune mice. J Exp Med. 1989 Oct 1;170(4):1439–1443. doi: 10.1084/jem.170.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Gahring L., Newton R., Daynes R. In vivo administration of interleukin 1 to normal mice depresses their capacity to elicit contact hypersensitivity responses: prostaglandins are involved in this modification of immune function. J Invest Dermatol. 1987 Apr;88(4):380–387. doi: 10.1111/1523-1747.ep12469071. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W., Bergstresser P. R. Genetic basis of ultraviolet-B effects on contact hypersensitivity. Immunogenetics. 1988;27(4):252–258. doi: 10.1007/BF00376119. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Bergstresser P. R., Streilein J. W. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980 Jan;124(1):445–453. [PubMed] [Google Scholar]
- Vermeer M., Schmieder G. J., Yoshikawa T., van den Berg J. W., Metzman M. S., Taylor J. R., Streilein J. W. Effects of ultraviolet B light on cutaneous immune responses of humans with deeply pigmented skin. J Invest Dermatol. 1991 Oct;97(4):729–734. doi: 10.1111/1523-1747.ep12484259. [DOI] [PubMed] [Google Scholar]
- Vincek V., Kurimoto I., Medema J. P., Prieto E., Streilein J. W. Tumor necrosis factor alpha polymorphism correlates with deleterious effects of ultraviolet B light on cutaneous immunity. Cancer Res. 1993 Feb 15;53(4):728–732. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Kurimoto I., Streilein J. W. Tumour necrosis factor-alpha mediates ultraviolet light B-enhanced expression of contact hypersensitivity. Immunology. 1992 Jun;76(2):264–271. [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Rae V., Bruins-Slot W., Van den Berg J. W., Taylor J. R., Streilein J. W. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990 Nov;95(5):530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Streilein J. W. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphism at the Tnfa and Lps loci governs susceptibility. Immunogenetics. 1990;32(6):398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]


