Abstract
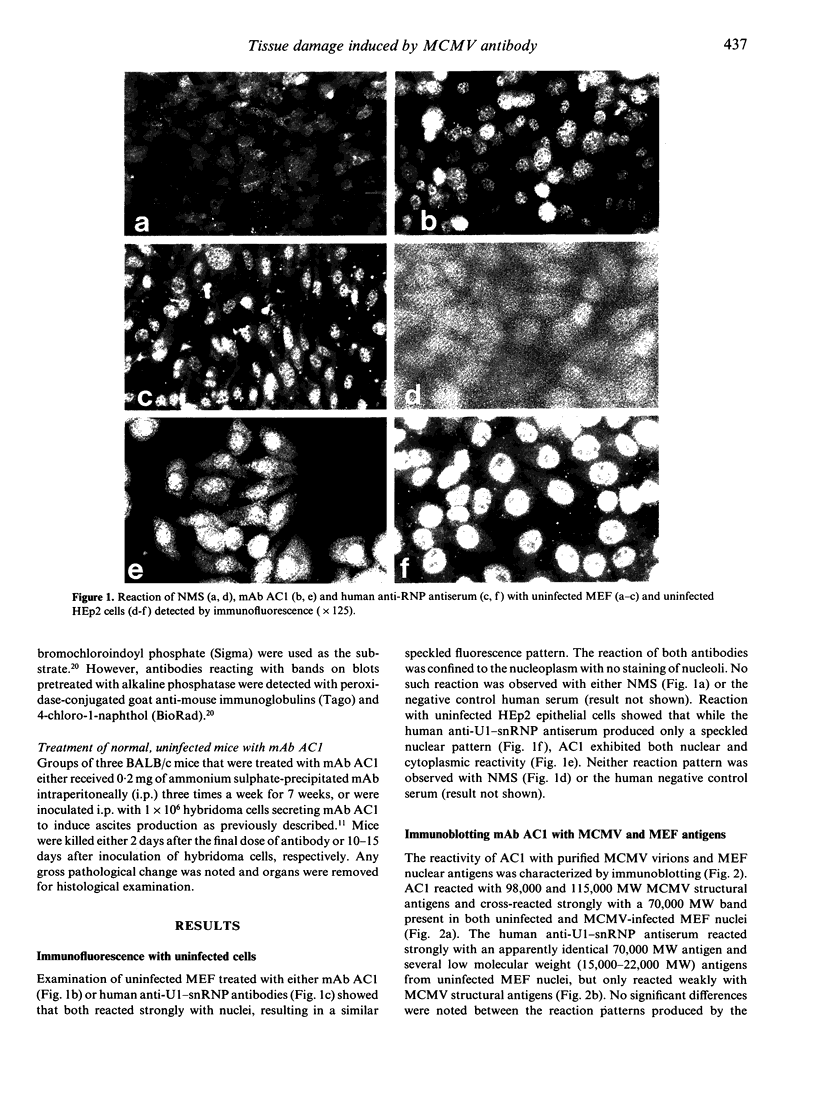
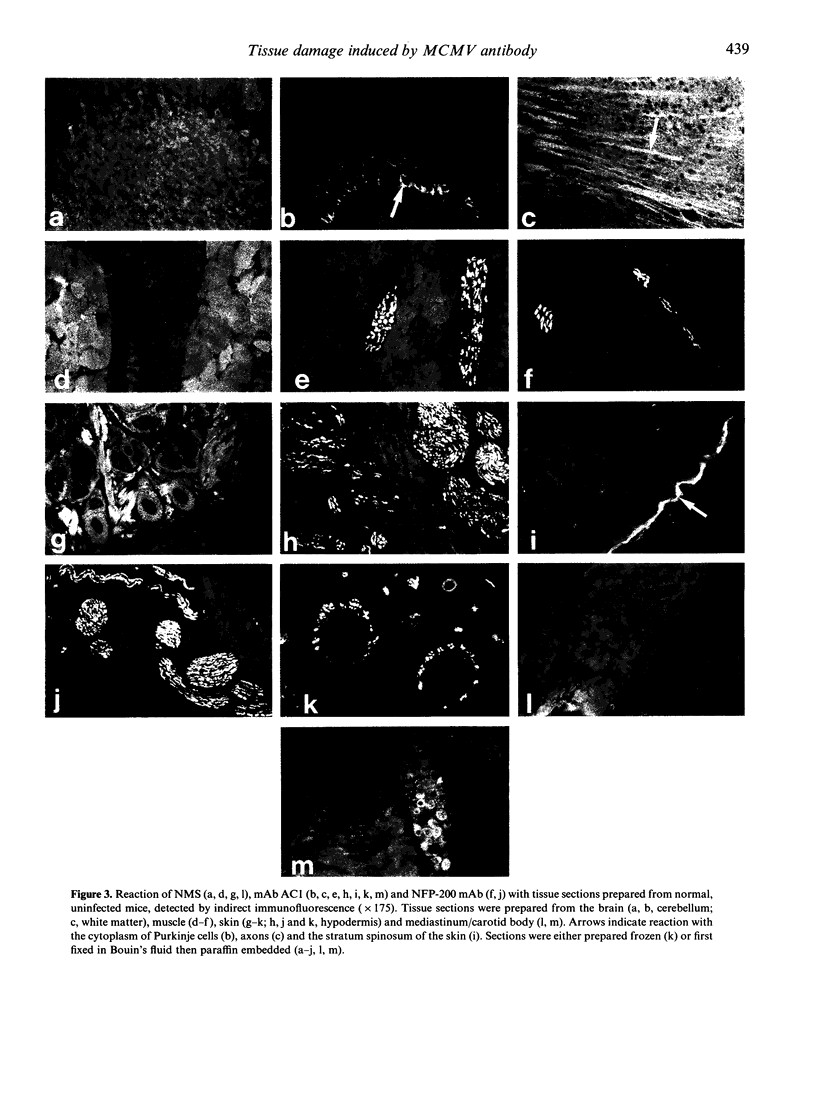
The autoreactivity of murine cytomegalovirus (MCMV)-neutralizing monoclonal antibody (mAb) AC1 was examined in vitro and in vivo. Both mAb AC1 and a human antiserum reactive with U1-small nuclear ribonucleoprotein (U1-snRNP) stained uninfected mouse embryo fibroblasts (MEF) in a speckled nuclear pattern and reacted with 70,000 molecular weight (MW) MEF nuclear antigens by immunoblotting, suggesting that mAb AC1 cross-reacted with the 70,000 MW component of U1-snRNP. However, only mAb AC1 cross-reacted with an additional epithelial cytoplasmic autoantigen present in cultured HEp2 cells. On tissue sections from uninfected mice, mAb AC1 predominantly reacted with a component of central and peripheral nervous systems, although cross-reactivity with the stratum spinosum of the skin and the outer sheath of hair follicles was also observed. Immunoblotting revealed that mAb AC1 reacted with phosphorylated epitopes present on a 98,000 MW MCMV structural protein and the 200,000 MW mouse neurofilament protein (NFP). Treatment of uninfected mice with mAb AC1 resulted in a severe interstitial pneumonia with greatly thickened and congested alveolar septa. Severe oedema of the hypodermis and a mild mesangial proliferative glomerulonephritis were also observed. These results demonstrate that a mAb reacting with a MCMV structural phosphoprotein which can protect mice against the dissemination of MCMV, can also promote the development of autoimmune disease. Therefore, the production of such cross-reactive antibodies may be an important mechanism in the development of autoimmunity following viral infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baboonian C., Venables P. J., Williams D. G., Williams R. O., Maini R. N. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. Ann Rheum Dis. 1991 Nov;50(11):772–775. doi: 10.1136/ard.50.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., O'Donoghue H., Foti D., Lawson C. M., Shellam G. R., Reed W. D. Multiple autoantibodies following cytomegalovirus infection: virus distribution and specificity of autoantibodies. Immunology. 1988 Jul;64(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., Shellam G. R., Allan J. E., Reed W. D., Joske R. A. Autoantibodies to liver-specific lipoprotein following hepatitis induced by mouse cytomegalovirus. Clin Exp Immunol. 1983 Apr;52(1):89–97. [PMC free article] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Boyce N. W., Fernando N. S., Neale T. J., Holdsworth S. R. Acute pulmonary and renal injury after administration of heterologous anti-lung antibodies in the rat. Characterization of ultrastructural binding sites, basement membrane epitopes, and inflammatory mediation systems. Lab Invest. 1991 Feb;64(2):272–278. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Satchwell S. C., Preddie E., Weston K. M., Barrell B. G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990 Apr 19;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Lee V. M. Dynamics of mammalian high-molecular-weight neurofilament subunit phosphorylation in cultured rat sympathetic neurons. J Neurosci Res. 1991 Sep;30(1):116–123. doi: 10.1002/jnr.490300113. [DOI] [PubMed] [Google Scholar]
- Cram D. S., Fisicaro N., Coppel R. L., Whittingham S., Harrison L. C. Mapping of multiple B cell epitopes on the 70-kilodalton autoantigen of the U1 ribonucleoprotein complex. J Immunol. 1990 Jul 15;145(2):630–635. [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Characterization of neutralizing monoclonal antibodies to murine cytomegalovirus. J Gen Virol. 1990 Mar;71(Pt 3):655–664. doi: 10.1099/0022-1317-71-3-655. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Immunoblot analysis of the antibody response to murine cytomegalovirus in genetically resistant and susceptible mice. J Gen Virol. 1989 Oct;70(Pt 10):2573–2586. doi: 10.1099/0022-1317-70-10-2573. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J Gen Virol. 1991 Jan;72(Pt 1):149–156. doi: 10.1099/0022-1317-72-1-149. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Lawley T. J., Hamburger M. I., Brown E. J. NIH Conference: Immunoglobulin G Fc receptor-mediated clearance in autoimmune diseases. Ann Intern Med. 1983 Feb;98(2):206–218. doi: 10.7326/0003-4819-98-2-218. [DOI] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Pages J., Bussard A., Goldberg M. A convenient enzyme-linked immunosorbent assay for testing whether monoclonal antibodies recognize the same antigenic site. Application to hybridomas specific for the beta 2-subunit of Escherichia coli tryptophan synthase. J Immunol Methods. 1983 Jun 10;60(3):351–358. doi: 10.1016/0022-1759(83)90292-2. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Shanley J. D., Griffiths P. D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987 Oct 31;2(8566):996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. A comparison of in vitro- and in vivo-phosphorylated neurofilament polypeptides. J Neurochem. 1981 Dec;37(6):1579–1585. doi: 10.1111/j.1471-4159.1981.tb06330.x. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini M. P., Lazzarotto T., Percivalle E., Ripalti A., Gerna G. Evidence that human cytomegalovirus assembly protein shares antigenic sites with an uninfected cell membrane protein. J Gen Virol. 1991 Dec;72(Pt 12):3009–3016. doi: 10.1099/0022-1317-72-12-3009. [DOI] [PubMed] [Google Scholar]
- Langley O. K., Sternberger N. H., Sternberger L. A. Expression of neurofilament proteins by Purkinje cells: ultrastructural immunolocalization with monoclonal antibodies. Brain Res. 1988 Aug 2;457(1):12–20. doi: 10.1016/0006-8993(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., Mitchell B. C., Henson P. M. The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Farrell H. E., Shellam G. R., Reed W. D. Murine anti-cytomegalovirus monoclonal antibodies with autoreactivity. Immunology. 1991 Mar;72(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Reed W. D. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992 Mar;75(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H. Myasthenia gravis induced by monoclonal antibodies to acetylcholine receptors. Nature. 1980 May 22;285(5762):238–240. doi: 10.1038/285238a0. [DOI] [PubMed] [Google Scholar]
- Ma J., Chapman G. V., Chen S. L., Melick G., Penny R., Breit S. N. Antibody penetration of viable human cells. I. Increased penetration of human lymphocytes by anti-RNP IgG. Clin Exp Immunol. 1991 Apr;84(1):83–91. doi: 10.1111/j.1365-2249.1991.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C., Caporali R., Negri C., de Gennaro F., Cerino A., Bestagno M., Cobianchi F., Astaldi-Ricotti G. C. Antibodies from patients with rheumatoid arthritis and systemic lupus erythematosus recognize different epitopes of a single heterogeneous nuclear RNP core protein. Possible role of cross-reacting antikeratin antibodies. Arthritis Rheum. 1990 Feb;33(2):180–186. doi: 10.1002/art.1780330205. [DOI] [PubMed] [Google Scholar]
- Price P., Eddy K. S., Papadimitriou J. M., Faulkner D. L., Shellam G. R. Genetic determination of cytomegalovirus-induced and age-related cardiopathy in inbred mice. Characterization of infiltrating cells. Am J Pathol. 1991 Jan;138(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- Price P., Hopkins R. M., Teo H. K., Papadimitriou J. M., Shellam G. R. Modulation of immunocompetence by cyclosporin A, cyclophosphamide or protein malnutrition potentiates murine cytomegalovirus pneumonitis. Pathol Res Pract. 1991 Dec;187(8):993–1000. doi: 10.1016/S0344-0338(11)81071-X. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Tolkoff-Rubin N. E., Oliver D., Rota T. R., Hamilton J., Betts R. F., Pass R. F., Hillis W., Szmuness W., Farrell M. L. Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation. 1985 Sep;40(3):243–249. doi: 10.1097/00007890-198509000-00004. [DOI] [PubMed] [Google Scholar]
- Schattner A., Rager-Zisman B. Virus-induced autoimmunity. Rev Infect Dis. 1990 Mar-Apr;12(2):204–222. doi: 10.1093/clinids/12.2.204. [DOI] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W., Geiger B. A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J. 1982;1(6):761–769. doi: 10.1002/j.1460-2075.1982.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley J. D., Pesanti E. L., Nugent K. M. The pathogenesis of pneumonitis due to murine cytomegalovirus. J Infect Dis. 1982 Sep;146(3):388–396. doi: 10.1093/infdis/146.3.388. [DOI] [PubMed] [Google Scholar]
- Shepp D. H., Dandliker P. S., de Miranda P., Burnette T. C., Cederberg D. M., Kirk L. E., Meyers J. D. Activity of 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine in the treatment of cytomegalovirus pneumonia. Ann Intern Med. 1985 Sep;103(3):368–373. doi: 10.7326/0003-4819-103-3-368. [DOI] [PubMed] [Google Scholar]
- Snary D., Flint J. E., Wood J. N., Scott M. T., Chapman M. D., Dodd J., Jessell T. M., Miles M. A. A monoclonal antibody with specificity for Trypanosoma cruzi, central and peripheral neurones and glia. Clin Exp Immunol. 1983 Dec;54(3):617–624. [PMC free article] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Autoantibodies and autoantigens: a conserved system that may shape a primary immunoglobulin gene pool. Mol Immunol. 1991 Dec;28(12):1399–1412. doi: 10.1016/0161-5890(91)90042-i. [DOI] [PubMed] [Google Scholar]
- Van Helden P. D., Van Lill L., Bester A. J., Hoal-Van Helden E. Autoantibodies from patients with systemic lupus erythematosus can affect DNA and RNA synthesis in cultured cells. Biochim Biophys Acta. 1989 Nov 2;1009(2):137–142. doi: 10.1016/0167-4781(89)90092-4. [DOI] [PubMed] [Google Scholar]
- Vital A., Vital C., Julien J., Baquey A., Steck A. J. Polyneuropathy associated with IgM monoclonal gammopathy. Immunological and pathological study in 31 patients. Acta Neuropathol. 1989;79(2):160–167. doi: 10.1007/BF00294374. [DOI] [PubMed] [Google Scholar]
- Ward M. M., Dawson D. V., Kredich D. W., Pisetsky D. S. Expression of IgM and IgG autoantibodies in pediatric and adult systemic lupus erythematosus. Clin Immunol Immunopathol. 1990 May;55(2):273–284. doi: 10.1016/0090-1229(90)90103-w. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Xu J., Dallas P. B., Lyons P. A., Shellam G. R., Scalzo A. A. Identification of the glycoprotein H gene of murine cytomegalovirus. J Gen Virol. 1992 Jul;73(Pt 7):1849–1854. doi: 10.1099/0022-1317-73-7-1849. [DOI] [PubMed] [Google Scholar]