Abstract
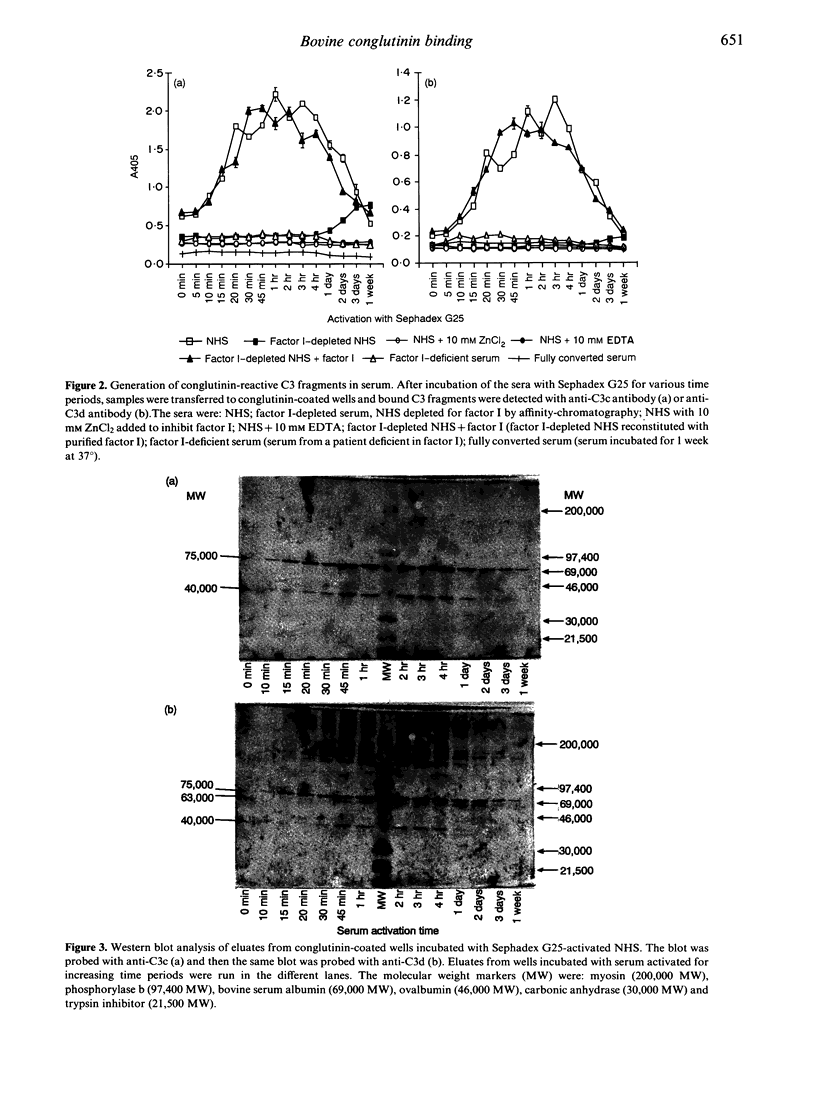
Bovine conglutinin is a serum lectin that agglutinates erythrocytes preincubated with antibodies and complement. This agglutination occurs through the binding of conglutinin to iC3b, a fragment of the complement component C3. It was reported that conglutinin binds fluid-phase C3b and C3c as well as iC3b. We re-investigated the reactivity of conglutinin towards fluid-phase C3 degradation products. ELISA wells were coated with conglutinin and reacted with C3 split products generated in normal human serum, in factor I-deficient serum, or in factor I-depleted serum. Conglutinin-bound C3 fragments were detected with anti-C3c and anti-C3d antibodies. An increased signal was observed during the activation of complement in normal human serum with the peak response after 1-2 hr, following which the signal decreased, reaching background level after 72 hr. The oligosaccharides on C3c, generated in serum, are thus not recognized by conglutinin. No signal was observed when factor I-deficient serum or factor I-depleted serum was used instead of normal serum. Reconstitution with purified factor I re-established the normal pattern. Examination of the conglutinin-bound C3 molecules by SDS-PAGE and Western blotting with anti-C3c and anti-C3d antibodies revealed bands characteristic for iC3b, and no bands corresponding to C3b or C3c. Reduction of the disulphide bonds prior to the incubation of the activated serum with the conglutinin-coated wells revealed a band of 63,000 MW, characteristic of the N-terminal fragment of the alpha-chain of iC3b. We also investigated the binding to the solid-phase conglutinin of purified C3 and degradation products generated with enzymes. In this case, C3 as well as C3b and C3c were bound, suggesting conformational changes in C3 during purification. In conclusion, when C3 conversion takes place at near physiological conditions, conglutinin interacts specifically with the oligosaccharide on the alpha-chain of iC3b.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Hartley C. A., Jackson D. C. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Gaither T. A., Hammer C. H., Hosea S. W., Frank M. M. The use of conglutinin in a quantitative assay for the presence of cell-bound C3bi and evidence that a single molecule of C3bi is capable of binding conglutinin. J Immunol. 1982 Feb;128(2):860–865. [PubMed] [Google Scholar]
- Dodds A. W. Small-scale preparation of complement components C3 and C4. Methods Enzymol. 1993;223:46–61. doi: 10.1016/0076-6879(93)23037-n. [DOI] [PubMed] [Google Scholar]
- Du Plessis J. L. The natural resistance of cattle to artificial infection with Cowdria ruminantium: the role played by conglutinin. Onderstepoort J Vet Res. 1985 Dec;52(4):273–277. [PubMed] [Google Scholar]
- Folkersen J., Teisner B., Hindersson P., Svehag S. E. A method for preparation of immunosorbents by direct coupling of dissociated immune complexes. J Immunol Methods. 1980;32(4):327–338. doi: 10.1016/0022-1759(80)90025-3. [DOI] [PubMed] [Google Scholar]
- Fornstedt N., Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975 Sep 15;57(2):187–191. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- Friis-Christiansen P., Thiel S., Svehag S. E., Dessau R., Svendsen P., Andersen O., Laursen S. B., Jensenius J. C. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990 Apr;31(4):453–460. doi: 10.1111/j.1365-3083.1990.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Friis P., Svehag S. E., Andersen O., Gahrn-Hansen B., Leslie R. G. Conglutinin exhibits a complement-dependent enhancement of the respiratory burst of phagocytes stimulated by E. coli. Immunology. 1991 Dec;74(4):680–684. [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Hartley C. A., Jackson D. C., Anders E. M. Two distinct serum mannose-binding lectins function as beta inhibitors of influenza virus: identification of bovine serum beta inhibitor as conglutinin. J Virol. 1992 Jul;66(7):4358–4363. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S., Kikuchi N., Ikenaka T., Inoue K. Structures of sugar chains of the third component of human complement. J Biochem. 1985 Oct;98(4):863–874. doi: 10.1093/oxfordjournals.jbchem.a135366. [DOI] [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Localization of the conglutinin binding site on the third component of human complement. J Immunol. 1985 Feb;134(2):1105–1109. [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Structural analysis of the asparagine-linked oligosaccharides of human complement component C3. Biochem J. 1986 Jan 15;233(2):613–616. doi: 10.1042/bj2330613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM D. G. The conglutination phenomenon. XIII. In vivo interactions of conglutinin and experimental bacterial infection. Immunology. 1959 Oct;2:322–333. [PMC free article] [PubMed] [Google Scholar]
- INGRAM D. G. The conglutination phenomenon. XIV. The resistance enhancing effect of conglutinin and immuno-conglutinin in experimental bacterial infections. Immunology. 1959 Oct;2:334–345. [PMC free article] [PubMed] [Google Scholar]
- LEON M. A. Role of cations in conglutination and in formation of properdinzymosan complex from bovine serum. Proc Soc Exp Biol Med. 1957 Oct;96(1):202–204. doi: 10.3181/00379727-96-23432. [DOI] [PubMed] [Google Scholar]
- LEON M. A., YOKOHARI R. CONGLUTINATION: SPECIFIC INHIBITION BY CARBOHYDRATES. Science. 1964 Mar 20;143(3612):1327–1328. [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linscott W. D., Ranken R., Triglia R. P. Evidence that bovine conglutinin reacts with an early product of C3b degradation, and an improved conglutination assay. J Immunol. 1978 Aug;121(2):658–664. [PubMed] [Google Scholar]
- Mizuochi T., Loveless R. W., Lawson A. M., Chai W., Lachmann P. J., Childs R. A., Thiel S., Feizi T. A library of oligosaccharide probes (neoglycolipids) from N-glycosylated proteins reveals that conglutinin binds to certain complex-type as well as high mannose-type oligosaccharide chains. J Biol Chem. 1989 Aug 15;264(23):13834–13839. [PubMed] [Google Scholar]
- Nilsson B., Grossberger D., Nilsson Ekdahl K., Riegert P., Becherer D. J., Nilsson U. R., Lambris J. D. Conformational differences between surface-bound and fluid-phase complement-component-C3 fragments. Epitope mapping by cDNA expression. Biochem J. 1992 Mar 15;282(Pt 3):715–721. doi: 10.1042/bj2820715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Nilsson Ekdahl K., Avila D., Nilsson U. R., Lambris J. D. Neoantigens in complement component C3 as detected by monoclonal antibodies. Mapping of the recognized epitopes by synthetic peptides. Biochem J. 1990 May 15;268(1):55–61. doi: 10.1042/bj2680055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Teisner B., Brandslund I., Folkersen J., Rasmussen J. M., Poulsen L. O., Svehag S. E. Factor I deficiency and C3 nephritic factor: immunochemical findings and association with Neisseria meningitidis infection in two patients. Scand J Immunol. 1984 Oct;20(4):291–297. doi: 10.1111/j.1365-3083.1984.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Schröder H. C., Poznanovic S., Gasić M. J., Matthes E., Müller W. E. Inhibition of human immunodeficiency virus-1 infection by human conglutinin-like protein: in vitro studies. Jpn J Cancer Res. 1992 May;83(5):458–464. doi: 10.1111/j.1349-7006.1992.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H., Schröder H. C., Poznanovic S., Matthes E., Müller W. E. Human conglutinin-like protein inhibits infection by the human immunodeficiency virus-1 in vitro. Res Virol. 1992 Mar-Apr;143(2):97–99. doi: 10.1016/s0923-2516(06)80087-7. [DOI] [PubMed] [Google Scholar]
- Wakamiya N., Okuno Y., Sasao F., Ueda S., Yoshimatsu K., Naiki M., Kurimura T. Isolation and characterization of conglutinin as an influenza A virus inhibitor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1270–1278. doi: 10.1016/0006-291x(92)90440-v. [DOI] [PubMed] [Google Scholar]
- Young N. M., Leon M. A. The carbohydrate specificity of conglutinin and its homology to proteins in the hepatic lectin family. Biochem Biophys Res Commun. 1987 Mar 13;143(2):645–651. doi: 10.1016/0006-291x(87)91402-1. [DOI] [PubMed] [Google Scholar]