Abstract
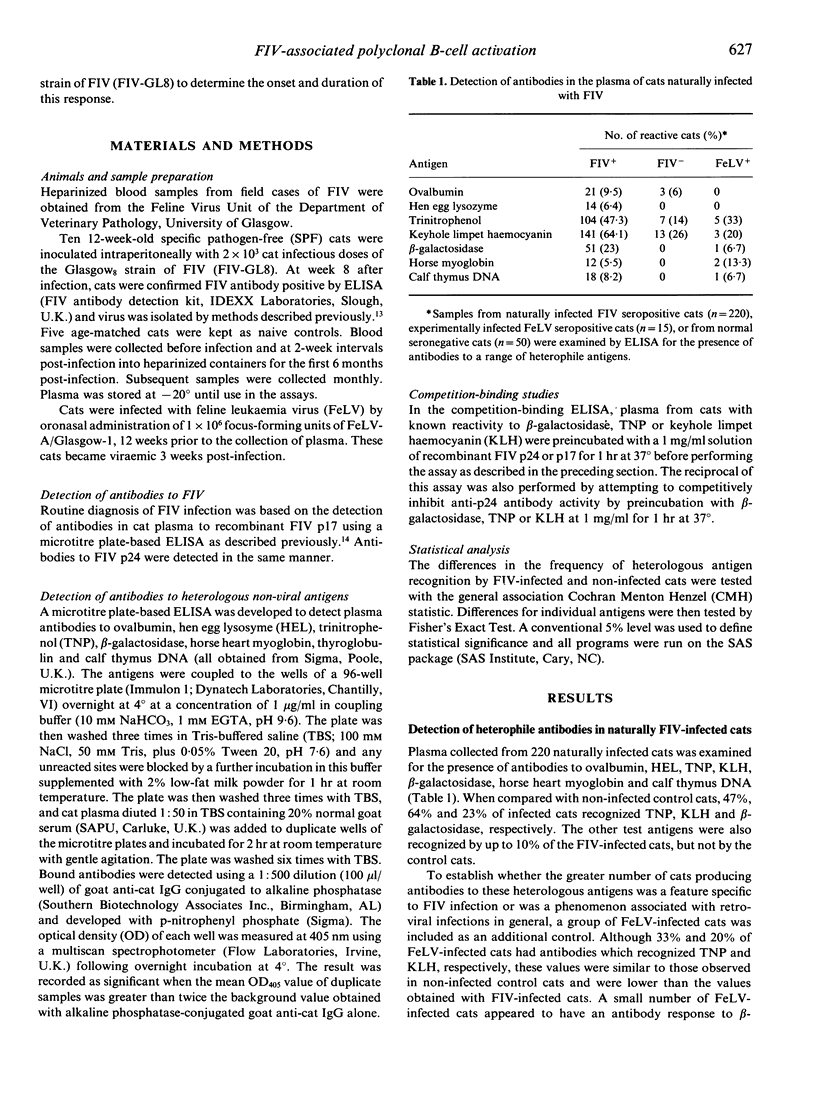
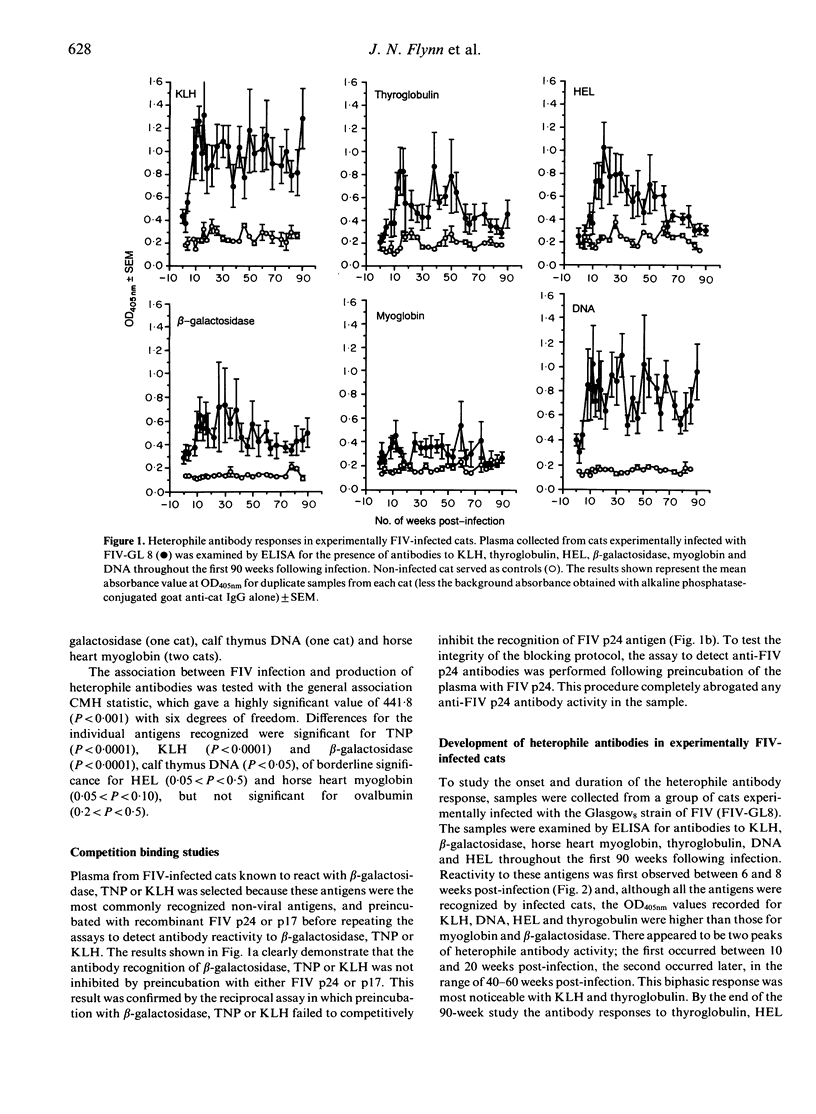
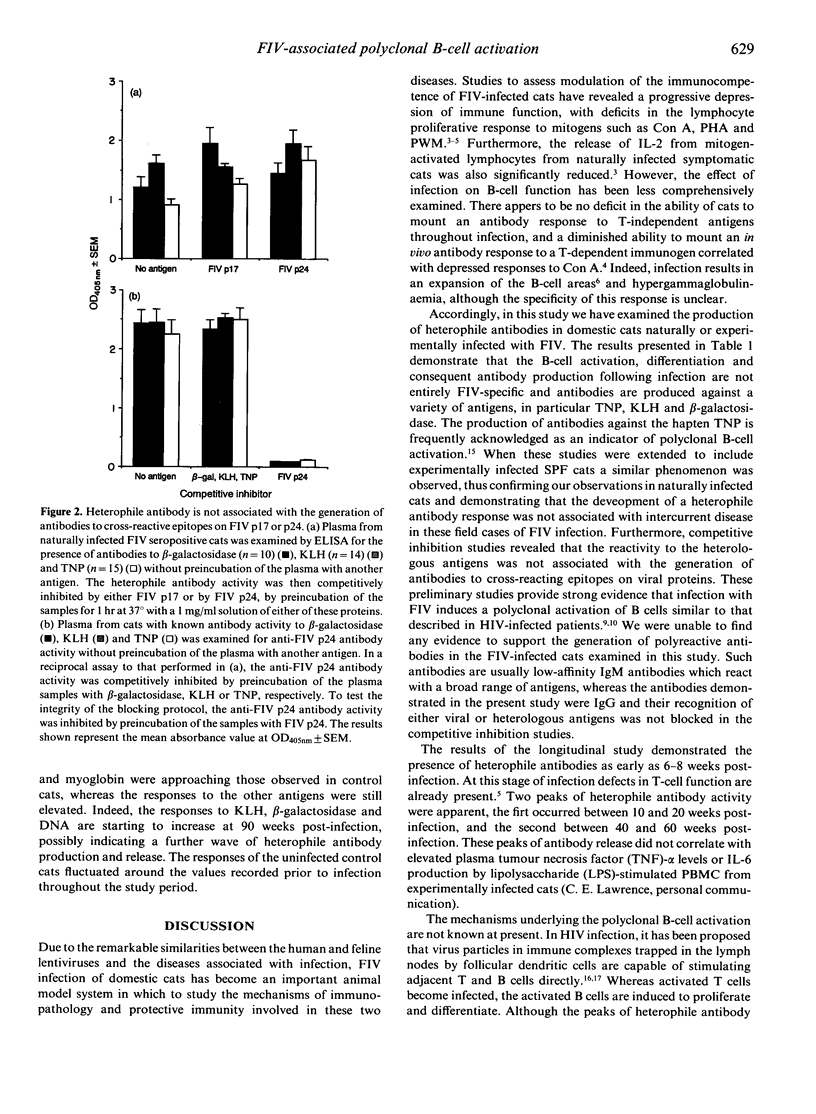
The specificity of the antibody response following natural or experimental infection of domestic cats with feline immunodeficiency virus (FIV) was examined. The antibody response to a range of non-viral antigens, including trinitrophenol (TNP), ovalbumin, beta-galactosidase, deoxyribonucleic acid (DNA) and keyhole limpet haemocyanin (KLH), was measured in 220 cats naturally infected with FIV. Infected cats had higher antibody levels to these antigens, in particular TNP, KLH and beta-galactosidase, than non-infected control cats. Competition binding studies demonstrated that this response was not due to the presence of cross-reacting epitopes on recombinant FIV p17 or p24 antigens, suggesting that the B-cell activation associated with infection was polyclonal rather than entirely virus specific. Studies on cats experimentally infected with FIV revealed a similar pattern, with infected cats developing an antibody response to heterologous non-viral antigens at 6-8 weeks post-infection. There were two discernible peaks of antibody activity, the first occurring 10-20 weeks post-infection and the second peak 40-60 weeks post-infection. The antibody response to KLH, DNA and beta-galactosidase remained elevated throughout the 90-week study period, whereas the antibody levels to the other antigens declined to levels approaching those observed in normal cats.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori A., Zamarchi R., Ciminale V., Del Mistro A., Siervo S., Alberti A., Colombatti M., Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989 Oct 1;143(7):2146–2152. [PubMed] [Google Scholar]
- Ammann A. J., Abrams D., Conant M., Chudwin D., Cowan M., Volberding P., Lewis B., Casavant C. Acquired immune dysfunction in homosexual men: immunologic profiles. Clin Immunol Immunopathol. 1983 Jun;27(3):315–325. doi: 10.1016/0090-1229(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Bennett M., Smyth N. R. Feline immunodeficiency virus: a brief review. Br Vet J. 1992 Sep-Oct;148(5):399–412. doi: 10.1016/0007-1935(92)90027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue F., Wallon C., Goujard C., Barre-Sinoussi F., Galanaud P., Delfraissy J. F. HIV induces IL-6 production by human B lymphocytes. Role of IL-4. J Immunol. 1992 Jun 15;148(12):3761–3767. [PubMed] [Google Scholar]
- Callanan J. J., McCandlish I. A., O'Neil B., Lawrence C. E., Rigby M., Pacitti A. M., Jarrett O. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection [corrected]. Vet Rec. 1992 Apr 4;130(14):293–295. doi: 10.1136/vr.130.14.293. [DOI] [PubMed] [Google Scholar]
- Callanan J. J., Thompson H., Toth S. R., O'Neil B., Lawrence C. E., Willett B., Jarrett O. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet Immunol Immunopathol. 1992 Dec;35(1-2):3–13. doi: 10.1016/0165-2427(92)90116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Hosie M. J., Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990 Mar;4(3):215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- Kopelman R. G., Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988 Jan;84(1):82–88. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- Laman J. D., Rácz P., Tenner-Rácz K., Klasmeier M., Fasbender M. J., Neelen C., Zegers N. D., Dietrich M., Boersma W. J., Claassen E. Immunocytochemical determination of antigen and epitope specificity of HIV-1-specific B cells in lymph-node biopsies from HIV-1-infected individuals. AIDS. 1991 Mar;5(3):255–262. doi: 10.1097/00002030-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lawrence C. E., Callanan J. J., Jarrett O. Decreased mitogen responsiveness and elevated tumor necrosis factor production in cats shortly after feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1992 Dec;35(1-2):51–59. doi: 10.1016/0165-2427(92)90120-f. [DOI] [PubMed] [Google Scholar]
- Macchia D., Almerigogna F., Parronchi P., Ravina A., Maggi E., Romagnani S. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993 Jun 3;363(6428):464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Reid G., Rigby M. A., McDonald M., Hosie M. J., Neil J. C., Jarrett O. Immunodiagnosis of feline immunodeficiency virus infection using recombinant viral p17 and p24. AIDS. 1991 Dec;5(12):1477–1483. doi: 10.1097/00002030-199112000-00010. [DOI] [PubMed] [Google Scholar]
- Shelton G. H., Grant C. K., Cotter S. M., Gardner M. B., Hardy W. D., Jr, DiGiacomo R. F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988). J Acquir Immune Defic Syndr. 1990;3(6):623–630. [PubMed] [Google Scholar]
- Shelton G. H., McKim K. D., Cooley P. L., Dice P. F., Russell R. G., Grant C. K. Feline leukemia virus and feline immunodeficiency virus infections in a cat with lymphoma. J Am Vet Med Assoc. 1989 Jan 15;194(2):249–252. [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J. E., George J. W., Mozes E., Lutz H., Pedersen N. C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991 May;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


