Abstract
Objective
To determine the precise in vivo interaction between T-cell costimulatory blockade and conventional immunosuppression in transplantation.
Summary Background Data
Blocking B7 or CD154 T-cell costimulatory activation pathways prevents allograft rejection in small and large animal transplant models and is considered a promising strategy for clinical organ transplantation.
Methods
A fully MHC-mismatched vascularized mouse cardiac allograft model was used to test the interactions between anti-CD154 or CTLA4Ig monotherapy and conventional immunosuppressive drugs in promoting long-term graft acceptance. The frequency of alloreactive T cell was measured by ELISPOT. Chronic rejection was examined by histology.
Results
Cyclosporine, tacrolimus, and anti-IL-2R monoclonal antibody therapy abrogated the effect of a single-dose protocol of anti-CD154 therapy. In contrast, rapamycin acted synergistically with anti-CD154 therapy in promoting long-term allograft survival. The addition of calcineurin inhibitors did not abolish this synergistic effect. Intense CD154-CD40 blockade by a multiple-dose schedule of anti-CD154 resulted in long-term graft survival and profound alloreactive T-cell unresponsiveness and overcame the opposite effects of calcineurin inhibitors. CTLA4Ig induced long-term graft survival, and the effect was not affected by the concomitant use of any immunosuppressive drugs.
Conclusions
The widespread view that calcineurin inhibitors abrogate the effects of T-cell costimulatory blockade should be revisited. Sufficient costimulatory blockade and synergy induced by CD154 blockade and rapamycin promote allograft tolerance and prevent chronic rejection.
Blocking T-cell costimulatory activation pathways is an effective strategy in preventing allograft rejection, promoting long-term survival, and inducing tolerance in some experimental transplant models. 1–8 The mechanisms of action of T-cell costimulatory blockade in vivo include induction of T-cell anergy, apoptosis, regulatory cells, and immune deviation. 9,10 Recent studies also demonstrated the efficacy of CD28-B7 and CD154-CD40 blockade in prolonging primate renal and islet allograft survival as relevant preclinical models for future translation to humans. 11–15 Furthermore, costimulatory blockade has been extensively studied as a promising therapeutic strategy not only in transplantation but also in autoimmunity, allergy, and infections. 9,16 Indeed, the efficacy of CTLA4Ig therapy has already been proven clinically in the autoimmune disease psoriasis vulgaris. 17–19 Phase I-II studies are underway with CTLA4Ig, humanized anti-B7, and anti-CD154 monoclonal antibodies (mAbs) in transplantation and autoimmunity.
One of the major challenges to developing T-cell costimulatory blockade strategies for the clinic, especially in the transplant setting, is understanding the interactions between agents that block T-cell costimulation and conventional immunosuppressive drugs currently in clinical use. 20 This is an extremely important and clinically relevant issue since immunosuppressive drugs may abrogate, synergize with, or not affect the functions of such agents. Previous reports showed that cyclosporine but not rapamycin abrogated the effect of combined blockade of CD28-B7 (by CTLA4Ig) and CD154-CD40 (by anti-CD154 mAb) costimulatory pathways in rodent transplantation models. 6,21,22 Smiley et al. also reported the distinct effects of some immunosuppressive drugs on anti-CD154 mAb therapy and showed that cyclosporine and steroids but not rapamycin abrogated the effect of anti-CD154 mAb plus concomitant administration of donor cells in promoting long-term allograft survival in a mouse heart transplant model. 23 The effect of the immunosuppressive drugs on CD154 mAb therapy alone was not investigated in that study. Kirk et al. recently reported that the additional use of steroids or tacrolimus to humanized anti-CD154 mAb might have a detrimental effect on graft survival in a primate renal transplant model. 13 Addition of cyclosporine or rapamycin to CTLA4Ig was reported to enhance allograft survival in a class I MHC-mismatched skin transplant model. 24
In this study, we investigated systematically the interactions between T-cell costimulatory blockade (CTLA4Ig to block CD28-B7 or MR1 to block CD154-CD40) and the immunosuppressive agents cyclosporine, tacrolimus, rapamycin, steroids, and IL-2R mAb in vivo. We used a model of vascularized cardiac transplantation in a fully allogeneic mouse strain combination, C57BL/6 into BALB/c. Our data highlight the complex interactions between B7 or CD154 blockade on the one hand and immunosuppressive drugs on the other in acute and chronic rejection, and provide clinically relevant novel data to translate to large animals and humans.
METHODS
Transplantation Model
C57BL/6 (H-2b) and BALB/c (H-2d) mice aged 6 to 8 weeks were purchased from Taconic Farms (Germantown, NY). BALB/c mice were used as recipients and C57BL/6 mice as donors. The cardiac allografts were placed in an intraabdominal location, as previously described. 25 Graft function was assessed by palpation of the heartbeat. Rejection was determined by complete cessation of palpable beat and was confirmed by direct visualization after laparotomy. 26
Fusion Proteins, mAbs, and Immunosuppressive Drugs
Anti-CD154 mAb (MR1, a kind gift of Dr. R. Noelle) and anti-IL-2R mAb (PC61, a kind gift of Dr. L. Turka) were manufactured from their respective hybridomas by Bioexpress Cell Culture Services (West Lebanon, NH). Murine CTLA4Ig was a generous gift of Dr. R. Peach (Bristol Myers Squibb, Princeton, NJ). Cyclosporine (Novartis), methylprednisolone (Upjohn), and tacrolimus (Fujisawa) were obtained from the Brigham and Women’s Hospital pharmacy. Rapamycin was generously provided by Wyeth-Ayerst (Princeton, NJ). Cyclosporine, methylprednisolone, and tacrolimus were prepared as a 0.15- to 4-mg/mL stock solution in 0.9% saline. Rapamycin was prepared as a 0.2-mg/mL stock solution in ethanol and was suspended in a carboxymethylcellulose and polysorbate vehicle.
Treatment Protocols
Recipients received MR1 (single dose [sMR1]: 250 μg intraperitoneal on day 0 or multiple doses [mMR1]: 500 μg intraperitoneal on day 0 plus 250 μg intraperitoneal on days 2, 4, and 6); CTLA4Ig (250 μg intraperitoneal on day 2); cyclosporine (20 mg/kg intraperitoneal on days 0–3, days 0–7, or days 30–33); tacrolimus (1.5 mg/kg intraperitoneal on days 0–3, days 0–7, or days 30–33); methylprednisolone (20 mg/kg intraperitoneal on days 0–3 or 0–7); rapamycin (0.3 mg/kg intraperitoneal on days 0–3); anti-IL-2R mAb (500 μg intraperitoneal on day 0 plus 250 μg intraperitoneal on days 2, 4, and 6).
Morphology
Cardiac grafts from long-term survivors (>100 days, n = 3–6/group) were fixed in 10% buffered formalin, embedded in paraffin, coronally sectioned, and stained with hematoxylin-eosin (H&E) for evaluation of cellular infiltrates, Verhoeff’s elastin for vessel arteriosclerosis scoring, or Masson’s trichrome stain for evaluation of fibrosis by light microscopy. Arteriosclerosis was assessed using light microscopy and percentage luminal occlusion by intimal thickening determined using the scoring system as previously described. 27–29 In brief, a vessel score of 0 indicated less than 10% luminal occlusion, 1 indicated less than 20%, 2 indicated 20% to 40%, 3 indicated 40% to 60%, 4 indicated 60% to 80%, and 5 indicated 80% to 100% occlusion. Only vessels that were cut orthogonally and that displayed a clear internal elastic lamina were scored. All arteries were scored by blinded examiners. Matched trichrome-stained sections were also examined for the presence of interstitial fibrosis.
ELISPOT
IMMUNOSPOT plates (Cellular Technology Ltd., Cleveland, OH) were coated with 4 μg/mL rat antimouse IFN-γ capture mAb (R4–6A2) in sterile phosphate-buffered saline (PBS) overnight. The plates were then blocked for 1 hour with sterile PBS containing 1% BSA-Fraction V and washed three times with sterile PBS. Splenocytes (1 × 106 in 200 μL HL-1 medium containing 1% L-glutamine) were then placed in each well in the presence of 1 × 106 irradiated (3,000 rad) syngeneic or allogeneic splenocytes and cultured for 24 hours at 37°C in 5% CO2. After washing with PBS followed by PBS containing 0.05% Tween (PBST), 2 μg/mL biotinylated rat antimouse IFN-γ detection mAb (XMG1.2) was added overnight. The plates were then washed four times in PBST, followed by 2 hours of incubation with streptavidin horseradish peroxidase (Dako, Carpenteria, CA) diluted at 1:2,000 in PBS/1% BSA. After washing three times with PBST followed by PBS, the plates were developed using 800 μL 3-amino-9-ethylcarbazole (AEC; Sigma-Aldrich, St. Louis, MO; 10 mg dissolved in 1 mL N-N dimethylformamide) mixed in 24 mL 0.1 mol/L sodium acetate, pH 5.0, plus 12 μL H2O2. The resulting spots were counted on a computer-assisted enzyme-linked immunospot image analyzer (T Spot Image Analyzer; Cellular Technology Ltd., Cleveland, OH). All mAbs were purchased from Pharmingen (San Diego, CA).
Statistics
Graft survival was expressed graphically using the Kaplan-Meier method, and statistical differences in survival between the groups were assessed by the log-rank test. Statistical analysis relating to ELISPOT data were ascertained using the Student t test and the Mann-Whitney test.
RESULTS
Interaction Between CD154 mAb and Various Immunosuppressive Agents
First we tested the effect of immunosuppressive drugs on CD154 blockade with a single dose of MR1. A single injection of MR1 (250 μg on the day of transplant) in our model significantly prolonged allograft survival and resulted in indefinite graft survival in 50% of recipients (mean survival time 45.5 ± 5.1, n = 15), comparable to previous reports. 7,26 A short course (day 0–3) of calcineurin inhibitors, cyclosporine or tacrolimus, which when given alone had only a marginal effect on graft survival, abrogated the effect of CD154 blockade and significantly inhibited the prolongation of graft survival induced by sMR1 (Fig. 1). Anti-IL-2R mAb also abrogated the effect of CD154 blockade significantly (Fig. 2). We tested the effect of two protocols of methylprednisolone, day 0 to 3 and day 0 to 7. Although the prolonged course of methylprednisolone tended to antagonize the effect of sMR1, there was no statistically significant difference in allograft survival between the groups (Fig. 3). Furthermore, addition of methylprednisolone to sMR1 plus cyclosporine or sMR1 plus tacrolimus had no further effect on graft survival (mean survival times 22.6 ± 4.8 and 27.4 ± 4.6, respectively, n = 6/group). In sharp contrast, RPM synergized with sMR1 and prolonged graft survival indefinitely (>100 days) in all recipients (Fig. 4). Interestingly, this striking synergistic effect was not abolished by the additional use of cyclosporine or tacrolimus, and all grafts survived over 100 days, an effect that required CD154 blockade since grafts from recipients treated with similar protocols of rapamycin plus cyclosporine or rapamycin plus tacrolimus were rejected (mean survival times 20.3 ± 2.6 and 20.3 ± 3.8, respectively, n = 4/group) (Table 1).
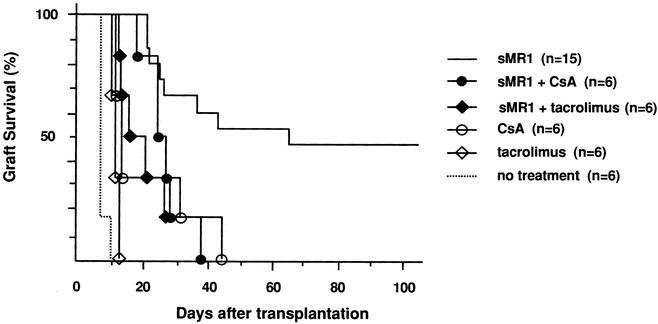
Figure 1. Effect of calcineurin inhibitors on anti-CD154 mAb therapy in the C57BL/6 into BALB/c cardiac transplant model. The concomitant use of cyclosporine or tacrolimus abrogated the effect of single-dose anti-CD154 mAb on cardiac allograft survival (P = .017 and P = .003, respectively, vs. sMR1 alone).
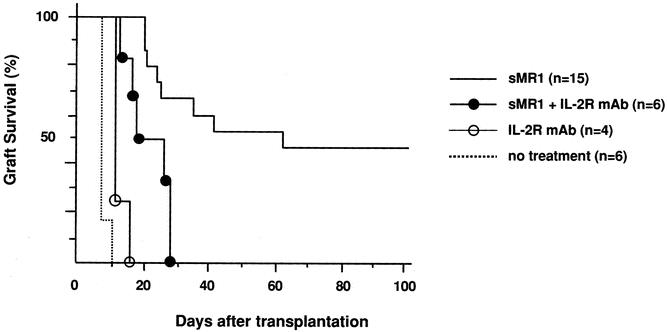
Figure 2. Effect of anti-IL-2R mAb on anti-CD154 mAb therapy in the C57BL/6 into BALB/c cardiac transplant model. The concomitant use of anti-IL-2R mAb abrogated the effect of single-dose anti-CD154 mAb on cardiac allograft survival (P = .0038 vs. sMR1 alone).
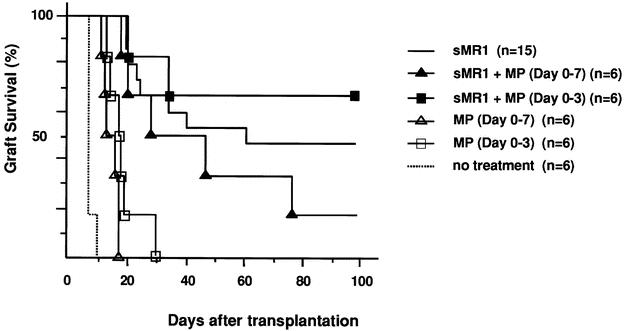
Figure 3. Effect of methylprednisolone on anti-CD154 mAb in the C57BL/6 into BALB/c cardiac transplant model. The concomitant use of neither a short (day 0–3) nor a long (day 0–7) course of methylprednisolone affected graft survival prolongation induced by a single dose of anti-CD154 mAb. There was no statistical significance among the groups of sMR1 with or without methylprednisolone.
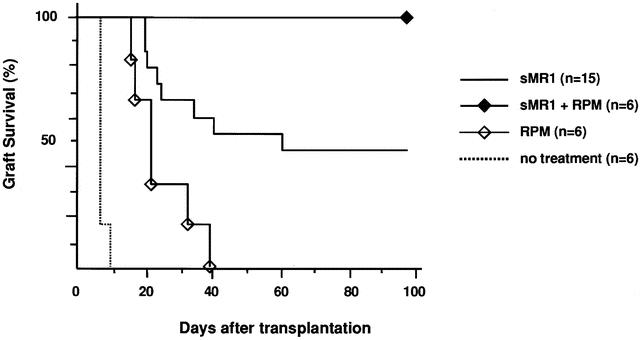
Figure 4. Effect of rapamycin on anti-CD154 mAb in the C57BL/6 into BALB/c cardiac transplant model. While rapamycin alone had only a marginal effect on allograft survival, it synergized with a single dose of anti-CD154 mAb to induce long-term graft survival in all recipients (P = .037 and P = .0005 compared to sMR1 alone and rapamycin alone, respectively).
Table 1. EFFECT OF CALCINEURIN INHIBITORS ON THE SYNERGY BETWEEN CD154 BLOCKADE AND RAPAMYCIN
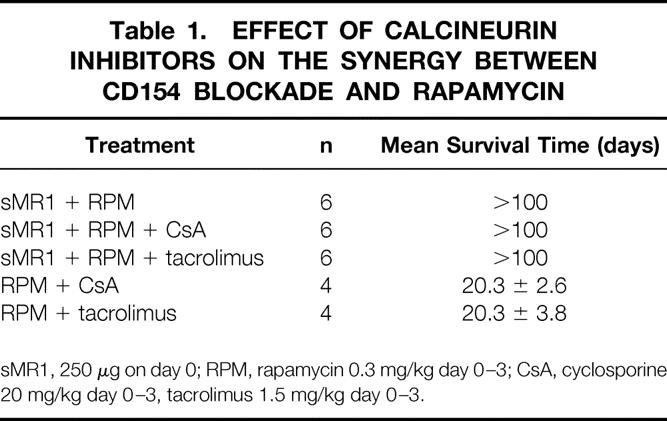
sMR1, 250 μg on day 0; RPM, rapamycin 0.3 mg/kg day 0–3; CsA, cyclosporine 20 mg/kg day 0–3, tacrolimus 1.5 mg/kg day 0–3.
The in vivo effects of rapamycin were also confirmed using a highly sensitive measure of donor-specific alloreactivity, the ELISPOT assay. 26 The frequency of IFN-γ-producing splenocytes in response to donor cells was measured on day 14 in unmodified control recipients and recipients treated with sMR1, rapamycin alone, and sMR1 plus rapamycin. Although sMR1 alone or rapamycin alone did not result in significant reduction in the frequency of IFN-γ-producing cells compared to unmodified control, sMR1 plus rapamycin resulted in a statistically significant reduction of IFN-γ-producing alloreactive T cells (Fig. 5).
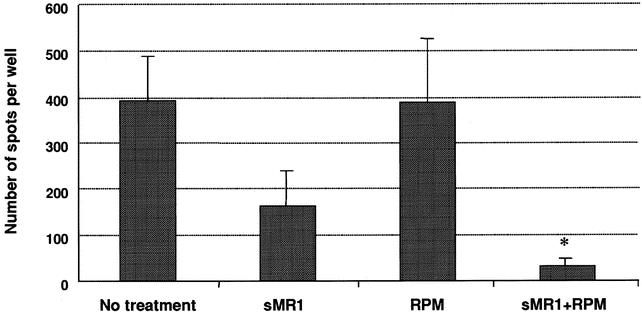
Figure 5. Frequency of IFN-γ-producing donor-specific splenocytes in recipients treated with none, a single dose of anti-CD154 mAb (sMR1), rapamycin, or sMR1 plus rapamycin following allogeneic heart transplantation. The results are expressed as the mean number of spots per well ± SEM obtained from three to six mice treated individually in each group. Control wells (medium or syngeneic) showed less than 10 spots per well. Therapy with sMR1 alone or rapamycin alone did not result in significant reduction of IFN-γ-producing T cells in response to donor cells as compared to untreated recipients. However, the frequency in mice treated with sMR1 plus rapamycin was significantly lower compared to controls (*P = .0095).
Furthermore, histologic analysis revealed that sMR1 plus rapamycin attenuated the development of chronic rejection (Fig. 6). Approximately 50% of mice treated with sMR1 alone accepted heart allografts for over 100 days. In those long-term allograft survivors, moderately severe chronic rejection, defined by development of allograft vasculopathy and extensive fibrosis, was observed. In most of these samples, vessels were difficult to evaluate because of the extensive fibrosis. By contrast, sMR1 plus rapamycin therapy attenuated the development of chronic allograft vasculopathy and fibrosis.
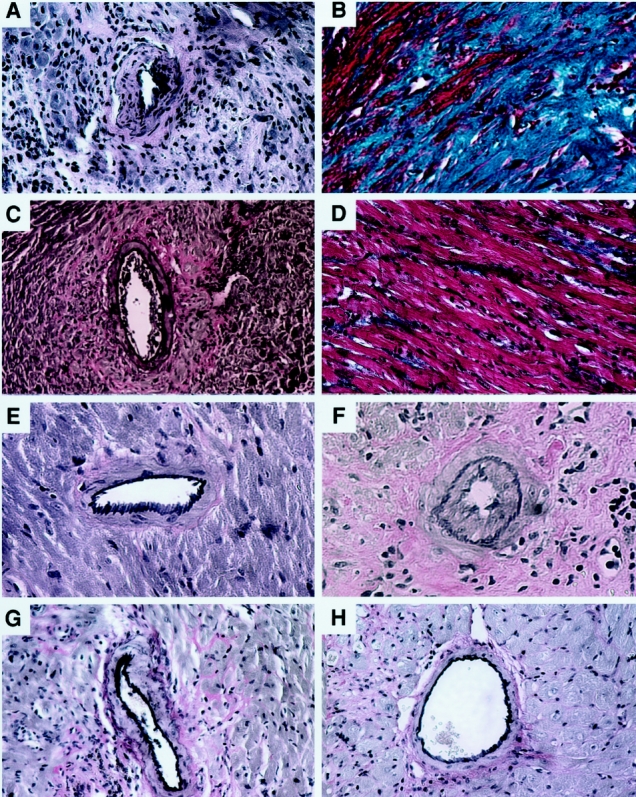
Figure 6. Effect of various therapies on the development of chronic allograft vasculopathy. Representative sections of mouse cardiac grafts at more than 100 days posttransplant from the following treatment groups (n = 4–8/group): (A) sMR1 (Verhoeff’s elastin stain, vessel score = 2.53 ± 1.94); (B) sMR1 (Masson’s trichrome stain); (C) sMR1 plus rapamycin (Verhoeff’s elastin stain, vessel score = 1.25 ± 1.25); (D) sMR1 plus rapamycin (Masson’s trichrome stain); (E) mMR1 (Verhoeff’s elastin stain, vessel score = 0.81 ± 0.53); (F) mMR1 plus late (day 30–33) cyclosporine (Verhoeff’s elastin stain, vessel score = 3.05 ± 0.69); (G) CTLA4Ig (Verhoeff’s elastin stain, vessel score 0.7 ± 0.41); (H) control isograft (Verhoeff’s elastin stain; vessel score = 0). Vessel scores are given as mean ± SEM. Original magnification ×200.
We were intrigued with the observation that cyclosporine or tacrolimus, which completely abrogated the effect of sMR1, did not abrogate the effect of sMR1 plus rapamycin, a regimen that resulted in indefinite survival of cardiac allografts in all recipients (see Fig. 4 and Table 1). Therefore, we hypothesized that an optimal protocol of CD154 blockade may overcome the effects of cyclosporine or tacrolimus. For that purpose we first tested the effect of a multiple-dose regimen of CD154 blockade on allograft survival in our model. This regimen has been previously published by our group and was shown to promote long-term graft survival and tolerance. 26 Indeed, all recipients treated with four doses of MR1 (mMR1; 500 μg on day 0 followed by 250 μg on day 2, 4, and 6) had long-term allograft survival of more than 100 days (Table 2). This therapy was also associated with profound T-cell hyporesponsiveness, as evidenced by marked reduction in the frequency of IFN-γ-producing alloreactive splenocytes (Fig. 7), comparable to the effect of sMR1 plus rapamycin (see Fig. 5). Furthermore, this treatment regimen with multiple MR1 doses also significantly prevented the development of chronic allograft vasculopathy (see Fig. 6). Interestingly, the concomitant use of calcineurin inhibitors (cyclosporine or tacrolimus, day 0–3), using the same regimen that abrogated the sMR1 effect, did not affect graft survival prolongation (see Table 2). We then tested the effect of a longer course of cyclosporine or tacrolimus (day 0–7) to make certain that calcineurin inhibitors were present at the same time as administration of MR1 (day 0–6). Again, calcineurin inhibitors had no effect on graft survival (see Table 2). Furthermore, additional methylprednisolone (day 0–3) to mMR1 plus cyclosporine or mMR1 plus tacrolimus had no further effect on graft survival (see Table 2).
Table 2. EFFECT OF CALCINEURIN INHIBITORS AND METHYLPREDNISOLONE ON MULTIPLE-DOSE REGIMEN OF CD154 BLOCKADE
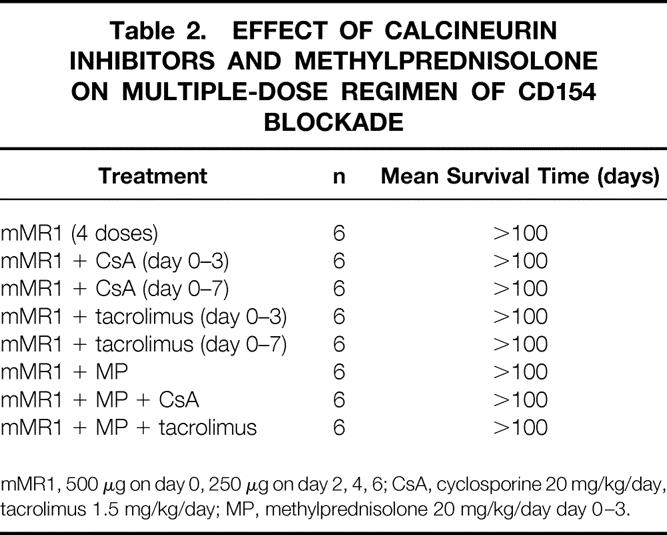
mMR1, 500 μg on day 0, 250 μg on day 2, 4, 6; CsA, cyclosporine 20 mg/kg/day, tacrolimus 1.5 mg/kg/day; MP, methylprednisolone 20 mg/kg/day day 0–3.
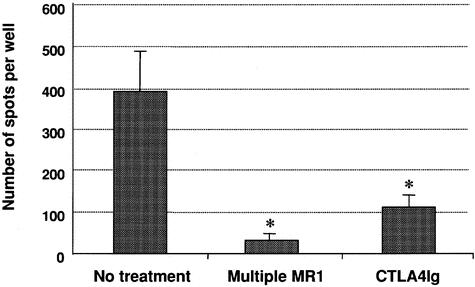
Figure 7. Frequency of IFN-γ-producing donor-specific splenocytes in recipients treated with none, multiple doses of anti-CD154 mAb (mMR1), or CTLA4Ig following allogeneic heart transplantation. The results are expressed as the mean number of spots per well ± SEM obtained from three to six mice treated individually in each group. Control wells (medium or syngeneic) showed less than 10 spots per well. *P = 0.0238 (mMR1 vs. controls) and .0095 (CTLA4Ig vs. controls), respectively.
Finally, since anti-CD154 therapy may be developed clinically as a relatively short-course induction therapy followed by introduction of immunosuppressive drugs, we tested the effect of cyclosporine on long-term graft survival when administered late after transplantation. For that purpose we used cyclosporine on day 30 to 33 posttransplantation in recipients treated with mMR1. Interestingly, although graft survival was not affected (>100 days, n = 6), histologic analysis of long-term surviving allografts revealed that the late (day 30–33) but not early introduction of cyclosporine promoted the development of chronic allograft vasculopathy (see Fig. 6).
Interaction Between CTLA4Ig and Various Immunosuppressive Agents
In our model, a single injection of CTLA4Ig on the second day after transplantation induced long-term survival, as previously published in the same model. 26 This treatment also induced profound hyporesponsiveness as determined by ELISPOT analysis of IFN-γ-producing alloreactive T cells (see Fig. 7). The beneficial effect of CTLA4Ig was not affected by the additional use of any immunosuppressant (Table 3). In addition, animals treated with CTLA4Ig were protected from development of chronic allograft vasculopathy (see Fig. 6), and the addition of immunosuppression had no effect.
Table 3. EFFECT OF VARIOUS IMMUNOSUPPRESSIVE DRUGS ON CTLA4Ig
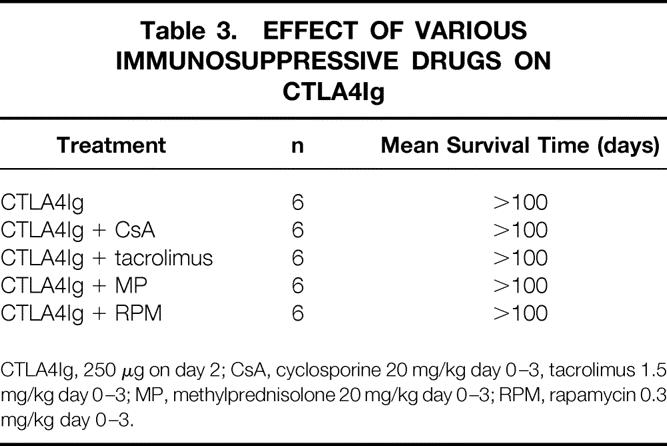
CTLA4Ig, 250 μg on day 2; CsA, cyclosporine 20 mg/kg day 0–3, tacrolimus 1.5 mg/kg day 0–3; MP, methylprednisolone 20 mg/kg day 0–3; RPM, rapamycin 0.3 mg/kg day 0–3.
DISCUSSION
Blockade of B7 or CD154 is a promising strategy for the prevention of allograft rejection and therapy of autoimmune diseases. 9,16 Since agents to block either pathway are unlikely to be used as monotherapy in the transplant setting, it is critical that we define the interactions between agents that block T-cell costimulation on the one hand and immunosuppressive drugs on the other. Previous reports have shown that some calcineurin inhibitors abrogated the efficacy of combined B7 plus CD154 costimulatory blockade in a mouse skin transplant model. 6,21,22 Rapamycin, on the other hand, was reported to be synergistic with B7 plus CD154 blockade in promoting long-term murine skin allograft survival. 21,22 In the heart transplant model, cyclosporine and steroids but not rapamycin were reported to abrogate the effect of CD154 blockade with concomitant administration of donor spleen cells in promoting allograft tolerance. 23 The only report on the effects of conventional immunosuppressive drugs on anti-CD154 monotherapy comes from Kirk et al. in primates, showing worse renal allograft survival with addition of tacrolimus. 13 However, it was not clear whether this was due to graft rejection or drug toxicity. Administration of cyclosporine or rapamycin was reported to enhance the effect of CTLA4Ig in prolonging mouse skin allograft survival. 24 We used the mouse vascularized cardiac transplant model to systematically evaluate the interactions between various immunosuppressive drugs currently in clinical use on B7 or CD154 blockade. This model allows for the evaluation of graft survival as well as development of chronic rejection. Although the effect of costimulatory blockade is dependent on the species, strain, and model used, our studies highlight several important and novel observations for potential future applications to clinical transplantation. However, one must carefully consider the limitations of small animal studies when translating experimental results to the clinic.
First, we show that a very brief exposure to calcineurin inhibitors, cyclosporine or tacrolimus (day 0–3 posttransplant), clearly antagonizes the effect of suboptimal CD154 blockade by anti-CD154 mAb monotherapy in vivo. Li et al. showed that cyclosporine, by inhibiting IL-2 secretion, inhibited activation-induced cell death (AICD) induced by combined B7 and CD154 blockade in vivo. 22 This previous report may be supported by our novel finding that anti-IL-2R mAb also antagonized the effect of single-dose MR1. Although anti-IL-2R mAb has been recently introduced into clinical transplantation, the long-term efficacy has not been established yet. 30,31 Furthermore, very recent in vitro study showed that CD4+CD25+ cells were essential for the induction of tolerance to alloantigen through T-cell costimulatory blockade. 32 Taken together, IL-2R mAb may be an unfavorable agent to combine T-cell costimulatory blockade for future clinical transplantation. Cyclosporine is also known to inhibit CD154 expression on T cells, 33 and Smiley et al., on the other hand, showed that cyclosporine inhibited the effect of CD154 blockade plus donor cells by suppressing NF-κB activation-induced CD154 expression. 23 However, neither cyclosporine nor tacrolimus, even when administered concomitantly with the anti-CD154 mAb (day 0–3 or day 0–7) in our model, was capable of antagonizing the effect of optimal CD154 blockade by anti-CD154 monotherapy with multiple injections of the mAb (day 0–6). This multiple-dose regimen was associated with profound T-cell unresponsiveness, as evidenced by marked reduction in the frequency of IFN-γ-producing alloreactive T cells using ELISPOT. A possible explanation for this profound hyporesponsiveness may be the interaction between CD154-CD40 and CD28-B7 pathways. Indeed, the engagement of CD40 on antigen-presenting cells upregulates CD80/86, particularly CD80. 34,35 Thus, optimal CD154 blockade might also inhibit CD28 signaling, resulting in profound T-cell unresponsiveness. Our data clearly indicate that calcineurin inhibitors do not universally abrogate the effect of CD154 blockade in promoting long-term allograft survival. In humans, clinical development of humanized anti-CD154 mAb will likely follow a multidose regimen; thus, our data are highly clinically relevant, suggesting that calcineurin inhibitors can be safely used with anti-CD154 mAb therapy. There may be some caveats to such a conclusion. First is the novel observation that even a short exposure to cyclosporine late posttransplantation (day 30–33) promoted the development of chronic allograft vasculopathy. Thus, induction therapy with anti-CD154 mAb followed by delayed introduction of calcineurin inhibitors, as is the current practice of some induction therapy regimens, should be avoided. 20 Second, the half-life of anti-CD154 mAb in mice is longer than concomitantly administered calcineurin inhibitors. 36 Therefore, there is a possibility that the more prolonged use of calcineurin inhibitors might block the effect of CD154 blockade. In fact, we tried a 30-day course of calcineurin inhibitors with the multiple-dose regimen of anti-CD154 mAb (n = 6/group). However, this treatment protocol was toxic to the animals since half of treated mice died between 7 and 14 days after transplantation, presumably due to drug toxicity of calcineurin inhibitors (20 mg/kg cyclosporine and 1.5 mg/kg tacrolimus). In contrast, we saw no obvious toxicity in intense anti-CD154 mAb or CTLA4Ig treatment. Interestingly, however, the remaining animals (n = 3 in each group) still had functioning grafts more than 100 days posttransplant, suggesting that a prolonged course of calcineurin inhibitors did not abrogate the effect of a multiple-dose regimen of anti-CD154 mAb on graft survival.
A second novel and clinically relevant observation in our study is the clear synergy between a suboptimal brief course of rapamycin and suboptimal CD154 blockade. Not only was there synergy in promoting long-term allograft survival, but also the addition of rapamycin overcame the antagonistic effect of calcineurin inhibitors on suboptimal CD154 blockade. Furthermore, the addition of rapamycin attenuated the development of chronic allograft vasculopathy and fibrosis observed with the suboptimal anti-CD154 therapy alone. CD154 deficiency or blockade does not protect against the development of chronic rejection. 37–39 Concomitant administration of donor cells or simultaneous blockade of B7 is required for the prevention of chronic rejection by CD154 blockade. 6,40 Interestingly, similar to the multiple-dose regimen of anti-CD154 mAb, the addition of rapamycin to single-dose MR1 also resulted in profound T-cell hyporesponsiveness of IFN-γ-producing alloreactive T cells. Both strategies were associated with resistance to calcineurin inhibitors and protection from chronic rejection. Long-term administration of rapamycin has been reported to halt the progression of chronic allograft vasculopathy in primates. 41 However, CD154 blockade alone is not sufficient to inhibit CD8+ alloreactive T cells or alloantibody production, 13,42 and that may be responsible for the development of chronic rejection. 39 Therefore, one potential explanation for our in vivo and ELISPOT results is that rapamycin may be effectively suppressing alloreactive CD8+ T-cell function by inhibiting signal transduction and cell cycle progression, 43,44 and/or effectively inhibiting alloantibody production. 45,46
Finally, CTLA4Ig-induced long-term allograft survival was not influenced by any immunosuppressive drugs in our model. This therapy was associated with T-cell hyporesponsiveness of IFN-γ-producing alloreactive T cells and protection from chronic rejection. Dai et al. previously reported that blocking the CD28-B7 T-cell costimulatory pathway with CTLA4Ig failed to induce long-term allograft survival in IL-2-deficient mice because of defective AICD. 47 Calcineurin inhibitors in our model did not affect allograft survival, suggesting that AICD was not completely inhibited by cyclosporine or tacrolimus therapy. Alternatively, CTLA4Ig has been shown to induce T-cell anergy, as evidenced by decreased clonal expansion and cytokine production, and thus AICD may not be the sole mechanism of action of CTLA4Ig in vivo. Indeed, a study by Tran et al. showed that the induction phase of tolerance by CTLA4Ig was caused by IL-2-responsive anergy, while the maintenance phase was caused by regulatory cells. 48 We have previously shown that induction of tolerance to renal allografts with CTLA4Ig was associated with Th2 switch, 5 although CTLA4Ig blocked both Th1 and Th2 alloimmune responses in vivo. 26 Collectively, these observations indicate that CD28-B7 blockade in vivo functions by various mechanisms depending on the model investigated. Furthermore, since in nonhuman primate models CTLA4Ig monotherapy has not been shown to induce indefinite graft survival or tolerance, 11,12 one must be careful in directly translating our data in small animals to humans. However, our current data suggest that clinical development of CTLA4Ig with either calcineurin inhibitors or rapamycin may be feasible.
In this study steroids did not synergize with or antagonize the effects of T-cell costimulatory blockade, although the prolonged course (day 0–7) was associated with a trend for worsening graft survival when administered with sMR1. Steroids have diverse biologic actions, 49 and since they have numerous unfavorable effects, 20 a steroid-free clinical protocol of T-cell costimulatory blockade would be desirable.
In conclusion, this study reveals several novel and important interactions between CD154 or B7 costimulatory blockade and immunosuppressive drugs. The widespread view that calcineurin inhibitors abrogate the effects of T-cell costimulatory blockade should be revisited. Intense costimulatory blockade and synergy induced by CD154 blockade and rapamycin may promote allograft tolerance and prevent chronic rejection.
Footnotes
Supported by National Institutes of Health grants RO1 AI-34965 and PO1 AI-41521.
Correspondence: Mohamed H. Sayegh, MD, Laboratory of Immunogenetics and Transplantation, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA.
E-mail: msayegh@rics.bwh.harvard.edu
Accepted for publication February 18, 2002.
References
- 1.Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA 1992; 89: 11102–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin H, Bolling SF, Linsley PS, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med 1993; 178: 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson TC, Alexander DZ, Winn KJ, et al. Transplantation tolerance induced by CTLA4-Ig. Transplantation 1994; 57: 1701–1706. [PubMed] [Google Scholar]
- 4.Pearson T, Alexander D, Hendrix R, et al. CTLA4-Ig plus bone marrow induces long-term allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation 1995; 61: 997–1004. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh MH, Akalin E, Hancock WW, et al. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med 1995; 181: 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381: 434–438. [DOI] [PubMed] [Google Scholar]
- 7.Hancock WW, Sayegh MH, Zheng XG, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA 1996; 93: 13967–13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Alexander DZ, Hollenbaugh D, et al. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation 1996; 61: 4–9. [DOI] [PubMed] [Google Scholar]
- 9.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998; 338: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto K, Dong VM, Sayegh MH. The role of costimulatory molecules as targets for new immunosuppressives in transplantation. Curr Opin Urol 2000; 10: 57–62. [DOI] [PubMed] [Google Scholar]
- 11.Levisetti MG, Padrid PA, Szot GL, et al. Immunosuppressive effects of hCTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol 1997; 159: 5187–5191. [PubMed] [Google Scholar]
- 12.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA 1997; 94: 8789–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 1999; 5: 686–693. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci USA 1999; 96: 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenyon NS, Fernandez LA, Lehmann R, et al. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes 1999; 48: 1473–1481. [DOI] [PubMed] [Google Scholar]
- 16.Reiser H, Stadecker MJ. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med 1996; 335: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 17.Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest 1999; 103: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams JR, Kelley SL, Hayes E, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med 2000; 192: 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayegh MH. Finally, CTLA4Ig graduates to the clinic. J Clin Invest 1999; 103: 1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999; 353: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Zheng XX, Li XC, et al. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation 1998; 66: 1387–1388. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Li XC, Zheng XX, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med 1999; 5: 1298–1302. [DOI] [PubMed] [Google Scholar]
- 23.Smiley ST, Csizmadia V, Gao W, et al. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFκB: implications for tolerance induction. Transplantation 2000; 70: 415–419. [DOI] [PubMed] [Google Scholar]
- 24.Hale DA, Gottschalk R, Maki T, et al. Use of CTLA4-Ig in combination with conventional immunosuppressive agents to prolong allograft survival. Transplantation 1997; 64: 897–900. [DOI] [PubMed] [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 1973; 16: 343–350. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto K, Dong VM, Issazadeh S, et al. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest 2000; 106: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams DH, Tilney NL, Collins JJ Jr, et al. Experimental graft arteriosclerosis. I. The Lewis-to-F-344 allograft model. Transplantation 1992; 53: 1115–1119. [PubMed] [Google Scholar]
- 28.Russell ME, Hancock WW, Akalin E, et al. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest 1996; 97: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glysing-Jensen T, Raisanen-Sokolowski A, Sayegh MH, et al. Chronic blockade of CD28-B7-mediated T-cell costimulation by CTLA4Ig reduces intimal thickening in MHC class I and II incompatible mouse heart allografts. Transplantation 1997; 64: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 30.Vincenti F. The role of newer monoclonal antibodies in renal transplantation. Transplant Proc 2001; 33: 1000–1001. [DOI] [PubMed] [Google Scholar]
- 31.Eckhoff DE, McGuire B, Sellers M, et al. The safety and efficacy of a two-dose daclizumab (Zenapax) induction therapy in liver transplant recipients. Transplantation 2000; 69: 1867–1872. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PA, Lees CJ, Waldmann H, et al. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood 2001; 98: 467–474. [DOI] [PubMed] [Google Scholar]
- 33.Fuleihan R, Ramesh N, Horner A, et al. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest 1994; 93: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 1996; 273: 1862–1864. [DOI] [PubMed] [Google Scholar]
- 35.Grewal IS, Foellmer HG, Grewal KD, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 1996; 273: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 36.Foy TM, Shepherd DM, Durie FH, et al. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med 1993; 178: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu K, Schonbeck U, Mach F, et al. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol 2000; 165: 3506–3518. [DOI] [PubMed] [Google Scholar]
- 38.Ensminger SM, Spriewald BM, Witzke O, et al. Intragraft interleukin-4 mRNA expression after short-term CD154 blockade may trigger delayed development of transplant arteriosclerosis in the absence of CD8+ T cells. Transplantation 2000; 70: 955–963. [DOI] [PubMed] [Google Scholar]
- 39.Ensminger SM, Witzke O, Spriewald BM, et al. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation 2000; 69: 2609–2612. [DOI] [PubMed] [Google Scholar]
- 40.Hancock WW, Buelow R, Sayegh MH, et al. Antibody-induced transplant arteriosclerosis is prevented by graft expression of antioxidant and anti-apoptotic genes. Nat Med 1998; 4: 1392–1396. [DOI] [PubMed] [Google Scholar]
- 41.Ikonen TS, Gummert JF, Hayase M, et al. Sirolimus (rapamycin) halts and reverses progression of allograft vascular disease in non-human primates. Transplantation 2000; 70: 969–975. [DOI] [PubMed] [Google Scholar]
- 42.Iwakoshi NN, Mordes JP, Markees TG, et al. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol 2000; 164: 512–521. [DOI] [PubMed] [Google Scholar]
- 43.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol 1996; 14: 483–510. [DOI] [PubMed] [Google Scholar]
- 44.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol 1999; 162: 2775–2784. [PubMed] [Google Scholar]
- 45.Chen H, Luo H, Daloze P, et al. Long-term in vivo effects of rapamycin on humoral and cellular immune responses in the rat. Immunobiology 1993; 188: 303–315. [DOI] [PubMed] [Google Scholar]
- 46.Thomson AW, Propper DJ, Woo J, et al. Comparative effects of rapamycin, FK 506 and cyclosporine on antibody production, lymphocyte populations and immunoglobulin isotype switching in the rat. Immunopharmacol Immunotoxicol 1993; 15: 355–369. [DOI] [PubMed] [Google Scholar]
- 47.Dai Z, Konieczny BT, Baddoura FK, et al. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol 1998; 161: 1659–1663. [PubMed] [Google Scholar]
- 48.Tran HM, Nickerson PW, Restifo AC, et al. Distinct mechanisms for the induction and maintenance of allograft tolerance with CTLA4-Fc treatment. J Immunol 1997; 159: 2232–2239. [PubMed] [Google Scholar]
- 49.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol 2000; 18: 309–345. [DOI] [PubMed] [Google Scholar]


