Abstract
Background:
Primary duodenal gastrinomas are now recognized as a common etiology for patients with sporadic Zollinger Ellison Syndrome (ZES); however, the clinical and pathologic features of this condition and long-term outcome after operation are not well characterized.
Methods:
Between November 1982 and September 2000, 63 patients diagnosed with sporadic ZES underwent resection of a primary duodenal gastrinoma and regional nodal metastases with curative intent. Data from a prospectively maintained database were reviewed for clinical and pathologic parameters relating to primary tumor size, location, frequency of lymph node metastases, and disease-specific and disease-free survival.
Results:
There were 41 males and 22 females (mean age, 48.6 years). The majority of duodenal gastrinomas were in the first or second portions of the duodenum (83%). Tumor size ranged from 0.2 to 2.0 cm with 62% measuring less than 1.0 cm. Sixty percent of individuals had regional lymph node metastases identified primarily in proximity to the primary tumor. At a median 10-year follow-up, the overall disease-specific and disease-free survivals were 100% and 60%, respectively. Actuarial 10-year disease-free survival was significantly higher for patients without lymph node metastases versus those with lymph node metastases (78% versus 48%, P = 0.0137).
Conclusions:
Duodenal gastrinomas in patients with sporadic ZES are frequently small, most commonly located in the proximal duodenum, and associated with regional lymph node metastases in 60%. Disease-free survival is lower for patients with regional lymph node metastases suggesting that a more systematic lymphadenectomy to extirpate occult disease may be indicated in this group.
Zollinger Ellison Syndrome (ZES) is characterized by hypersecretion of gastrin from a neuroendocrine tumor resulting in profound gastric acid hypersecretion and a spectrum of clinical presentations including symptoms of refractory peptic ulcer disease, severe diarrhea, or intestinal perforation.1 In the past, surgical intervention consisted mainly of palliative gastrectomy to prevent the morbid consequences associated with uncontrolled gastric acid hypersecretion. With improvement in medical management using proton pump inhibitors, gastric acid hypersecretion can be effectively controlled in 100% of individuals in most series,2,3 which has effectively eliminated the need for emergent or palliative surgical intervention in patients diagnosed with the condition. Currently, surgical management is directed towards identification and resection of the primary tumor and regional metastases to lymph nodes or liver to prevent malignant progression of disease. In addition, cure as defined as 10-year biochemically confirmed disease-free survival can be achieved in 34% of patients with sporadic gastrinoma undergoing resection with curative intent.4
Primary duodenal gastrinomas are now recognized to be a more common etiology of sporadic ZES than previously thought and the use of routine duodenotomy during surgical exploration has resulted in improved identification and resection of these frequently very small primary tumors.5–7 Our institution has previously reported that small primary duodenal gastrinomas are associated with lymph node metastases in over 50% of individuals at operation,6 but the long-term outcome of patients with primary duodenal tumors and the significance of associated lymph node metastases are not known. The current study was undertaken to characterize our current results for patients who have sporadic gastrinomas arising in the duodenum who underwent resection with curative intent. Primary tumor size and location, the frequency and location of associated lymph node metastases, and overall and disease-free survival were analyzed.
METHODS
From November 1982 until September 2000, 63 patients with diagnosed ZES underwent resection of a primary duodenal gastrinoma for cure. The diagnosis of ZES was made by biochemical analysis consisting of the following studies performed in the absence of antisecretory medication: measurement of fasting serum gastrin level (FSG), the change in serum gastrin level after secretin stimulation, and the levels of basal acid output and maximal acid output. An abnormal fasting serum gastrin level was defined as a serum gastrin concentration greater than 100 pg/mL when measured before September 1994 and greater than 200 pg/mL when measured thereafter. A basal acid output greater than or equal to 15 mEq/h was abnormal if the patient had no previous acid reducing surgery or greater than 5 mEq/h if the patient had previous acid reducing surgery. An abnormal secretin stimulation test was defined as an incremental increase in serum gastrin level greater than 200 pg/mL after the intravenous administration of 2 U/kg of secretin. All patients had an abnormality in at least 2 or more of the above tests to make the diagnosis of ZES.
All patients underwent standard imaging studies to identify the location and extent of disease. Preoperative abdominal computed tomography (CT) with and without intravenous contrast, abdominal ultrasonography (US), and magnetic resonance imaging (MRI) were performed. Some patients also underwent preoperative portal venous sampling, secretin angiogram and somatostatin receptor scintigraphy depending on which institutional review board-approved protocol they were under and the availability of the test. Imaging studies were read by radiologists at our institution and individual reports were generated. Preoperative CT, MRI, and US reports for each patient were reviewed in a retrospective fashion to determine if individual radiographic tests were able to locate the duodenal primary found at operation.
Data collection and evaluation were performed at the National Institutes of Health. One patient underwent exploration at Walter Reed Army Medical Center, Bethesda, MD, 2 patients underwent operation at Barnes Hospital, St. Louis, MO, and 2 at the University of California, San Francisco, CA. All of the other patients underwent exploration at the National Institutes of Health, Bethesda, MD. Preoperative and postoperative evaluation for all patients was performed at the NIH. Patients were excluded from this study if they had any evidence of liver, lung or bone metastases present preoperatively.
Patients underwent operative exploration to localize and resect a primary gastrinoma and lymph node or other metastases. This was performed through a standard Chevron incision. The abdominal cavity was systematically explored for evidence of disease by examining the liver, pelvis, small intestine, large intestine, stomach, duodenum, pancreas, retroperitoneum, periportal lymph nodes, celiac axis lymph nodes, and periduodenal and peripancreatic lymph nodes. A Kocher maneuver was performed to fully mobilize the head of the pancreas and duodenum and the lesser sac was opened to examine the pancreatic body and tail. The duodenum and pancreas were carefully palpated between 2 fingers and any suspicious lesions were noted. Intraoperative ultrasonography (IOUS) was performed by the operating surgeon with help from a radiologist.8 The US probe was passed over the duodenum, pancreas, and liver. Again, any suspicious lesions were documented. Then, intraoperative upper gastrointestinal endoscopy was performed by a gastroenterologist. A standard adult gastroscope was passed through the patient’s mouth and guided through to the duodenum. Transillumination was performed as previously described.9 If a duodenal lesion was found by this method, the site at which it was located was marked with a simple stitch in the bowel wall. The ability of any of the above methods to localize the duodenal primary was recorded in the operative note. A longitudinal duodenotomy was performed in all patients for resection of the primary tumor as well as to localize 1 if the primary was not already located by 1 of the previously mentioned methods. If a primary tumor was not on the medial duodenal wall it was elliptically excised with a margin of 2 to 3 mm. If the primary tumor was located on the medial duodenal wall a separate submucosal excision was performed through the duodenotomy. The longitudinal duodenotomy was typically closed in a 2-layer transverse orientation to minimize narrowing of the lumen. Suspicious lymph nodes found at exploration by visualization, palpation, or US were also removed and sent to pathology. Their location was clearly documented in the operative note. If present, other benign appearing lymph nodes adjacent to pathologic nodes were also resected.
Pathologic diagnosis of a primary duodenal gastrinoma was made for all patients by immunohistochemical analysis for the presence of gastrin as well as histologic appearance. The size of the tumor was measured and the largest diameter was documented. Lymph nodes were evaluated in a similar manner and determined to be positive or negative for metastatic gastrinoma. Pathology reports were reviewed in a retrospective fashion for the size of the primary duodenal gastrinoma and the presence of positive lymph nodes. Based on the operative and pathology notes, the site at which positive lymph nodes were found was mapped.
After surgery, all patients were evaluated at an initial 3- to 6-month follow-up and then yearly. At each follow-up biochemical analysis (FSG, secretin stimulation test) and radiographic evaluation (CT, MRI, US) were performed. After 1994, it became standard to also evaluate patients with somatostatin scintigraphy yearly. Patients were determined to be cured of ZES if the following criteria were met: normal fasting serum gastrin level (<100 pg/mL before 1985, <200 pg/mL from 1985 on), less than a 200 pg/mL rise in serum gastrin after the administration of secretin (since 1998, evaluation with this test has been used selectively because of limitedness of secretin), and no radiographic evidence of disease by imaging studies. If a patient was initially considered cured, the follow-up date at which any of the above criteria were not met, was determined to be the recurrence date. Persistent disease was defined as continued abnormality on biochemical analysis or radiographic analysis with no period of normalization after the initial operation.
Statistical analyses were performed for the evaluation of disease-free survival and the influence of positive lymph nodes on disease-free survival. The disease-specific survival and overall survival were graphed by the Kaplan–Meier method. The influence of positive lymph nodes on survival after resection was analyzed by the Fisher exact test.
RESULTS
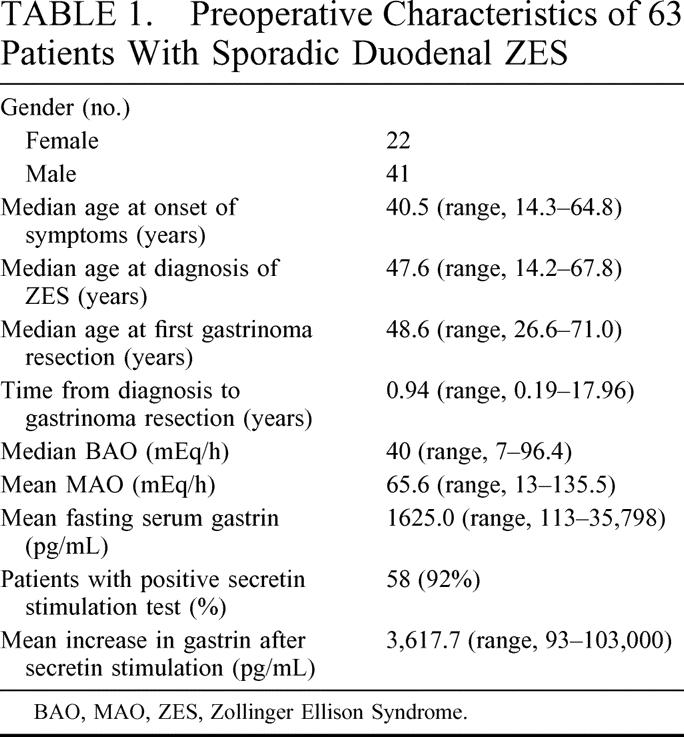
Sixty-three patients with sporadic ZES (22 females and 41 males) underwent resection of a duodenal primary gastrinoma with curative intent (Table 1). All patients were diagnosed with ZES based on biochemical tests including basal acid output (BAO), FSG, and secretin stimulation test. Preoperative FSG levels and gastrin levels after secretin stimulation were quite variable among the patients consistent with the differences in the degree of symptoms on presentation in this group. Five patients had evidence of ZES based on abnormal FSG levels, BAO, and maximal acid output (MAO) but had a normal secretin stimulation test. The majority of patients were diagnosed in the fifth decade with 1 patient presenting at the age of 67 and another at the age of 14 (Table 1). The time from diagnosis to surgical exploration was on average 1.3 years with 1 patient presenting 18 years after diagnosis. All patients underwent at least 1 operation for surgical resection of gastrinoma. Thirteen patients underwent more than 1 operation for either recurrent disease or inability to locate the primary tumor at the prior operations. A total of 77 operations were performed.
TABLE 1. Preoperative Characteristics of 63 Patients With Sporadic Duodenal ZES
All patients underwent CT of the abdomen preoperatively. Seven patients did not undergo a MRI as the test was not available at the time and 1 patient did not undergo an ultrasound prior to resection. Of note, primary duodenal gastrinomas were not imaged preoperatively by CT, MRI, or US in any patient. The inability to preoperatively localize a primary duodenal tumor with these imaging modalities was confirmed by review of the imaging reports for each patient. This highlights the importance of intraoperative methods, particularly duodenotomy, for diagnosis of a duodenal primary tumor (Table 2). All patients underwent intraoperative exploration with visualization and palpation. Forty-six patients underwent intraoperative US localization and 49 patients underwent intraoperative transillumination. Palpation was more sensitive (71% localization rate) than transillumination or US (59.2% and 43.5% localization rates, respectively). In 5 patients, palpation, US and transillumination were negative for locating the duodenal primary. Thus, these 5 patients were diagnosed solely by duodenotomy. One of these patients had a tumor in the fourth portion of the duodenum that potentially could easily be missed by transillumination and US. However, the other 4 had a duodenal primary in the first (3 patients) or second portions (1 patient) of the duodenum.
TABLE 2. Primary Duodenal Tumor Characteristics
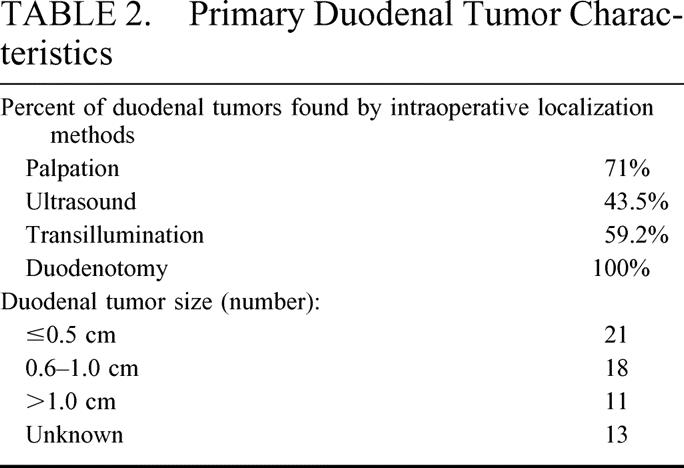
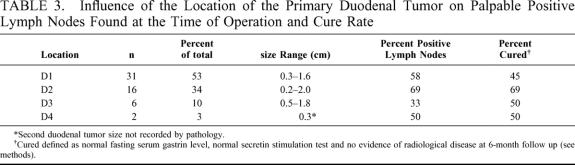
The primary tumor was located in the first and second portions of the duodenum in 83% of cases (Table 2, Fig. 1). A gastrinoma in the fourth portion of the duodenum was unusual (3.4%). Duodenal tumor size ranged from 0.2 to 2 cm in diameter with the majority less than 1 cm. Sixty percent of patients had lymph nodes pathologically positive for gastrinoma (Table 3). Of those patients with pathologically involved lymph nodes, 8 had these resected at a surgical exploration performed either before or after the operation performed for resection of the primary duodenal tumor. We determined the distribution of positive lymph nodes in relationship to the primary tumor. A map was constructed based on operative localization and pathologic findings of duodenal tumors and their associated lymph nodes (Fig. 2). The majority of lymph nodes were found to be located in the peripancreatic and periduodenal areas and appeared to be in proximity to the primary tumor. Thorough exploration was performed at the time of operation at various locations including the porta hepatis, celiac axis, superior mesenteric artery, peripancreatic and periduodenal lymph nodes independent of the location of the primary. Duodenal primary location as well as positive versus negative lymph nodes did not significantly influence immediate postoperative cure rate.
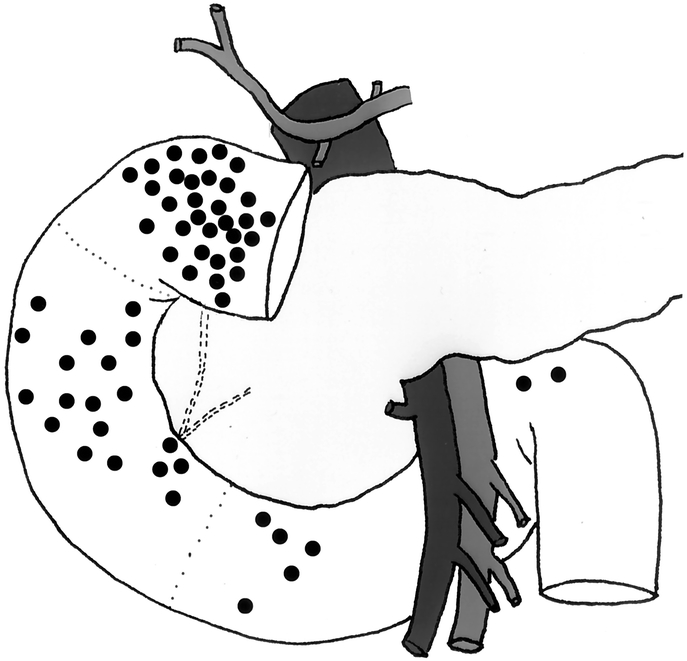
FIGURE 1. Location of primary tumors in 57 evaluable patients with sporadic duodenal ZES undergoing operation with curative intent. The first, second, and third/fourth portions of the duodenum are designated by dashed lines.
TABLE 3. Influence of the Location of the Primary Duodenal Tumor on Palpable Positive Lymph Nodes Found at the Time of Operation and Cure Rate
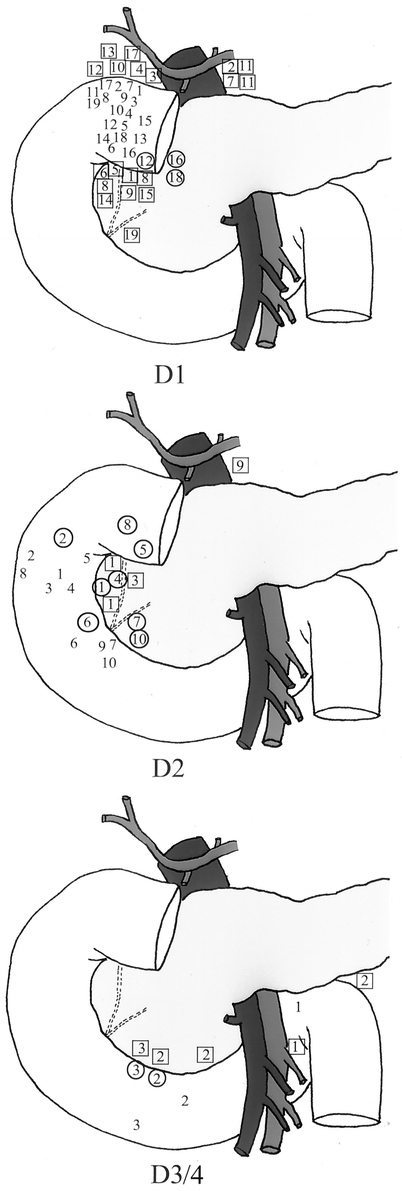
FIGURE 2. Location of lymph node metastases in relation to the primary location in 32 patients with sporadic duodenal ZES. Tumors and associated lymph node metastases for each portion of the duodenum are numbered. The corresponding lymph node metastases for each tumor are marked with a square if positioned anterior and within a circle if posterior to the duodenum.
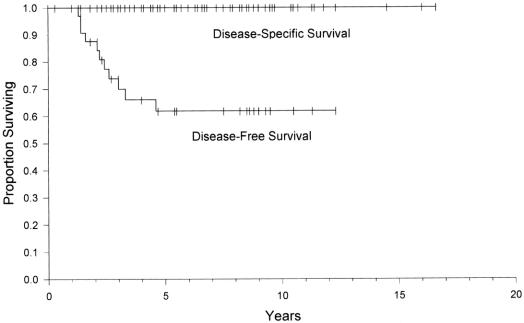
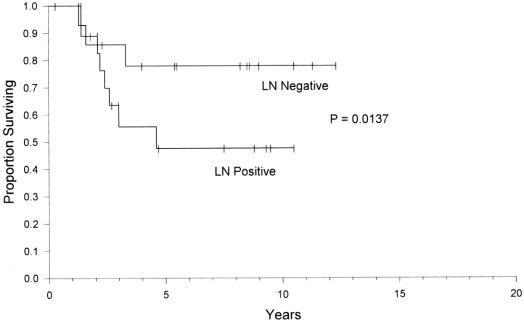
Cure was defined as a normal fasting serum gastrin, normal secretin stimulation test and no evidence of radiologic disease. During long-term follow-up there were 9 who died of causes unrelated to ZES. Thus, there was a 100% overall disease-specific survival rate illustrating the indolent nature of the disease. At a median potential follow-up of 10 years, the disease-free survival (biochemical cure) was 60% (Fig. 3). We evaluated the potential impact of nodal status on long-term outcome. A Kaplan–Meier plot of disease-free survival for patients following resection of the primary duodenal gastrinoma who had positive lymph nodes versus those who had no positive lymph nodes is shown in Figure 4. At 5 years of follow-up, less than 50% of patients who had resection of positive lymph nodes were still cured versus almost 80% for those with no positive lymph nodes. Thus, lymph node status appeared to be a statistically significant indicator for duration of disease-free interval after established cure (P = 0.014).
FIGURE 3. Disease-specific and disease-free survival for patients with sporadic duodenal gastrinoma following resection.
FIGURE 4. Disease-free survival in sporadic ZES patients with or without associated lymph node metastases.
DISCUSSION
Originally, duodenal wall gastrinomas were felt to be an uncommon cause of ZES.10 They were often missed, being found in the proximal duodenum of the total gastrectomy specimen by the pathologist after operation in up to 27% of cases.11 More recent published series have determined that the frequency of these tumors is much greater than previously reported occurring in 25 to 50% of patients operated on for ZES.6,11–13 These tumors are often small (<1 cm), making them difficult to locate preoperatively and intraoperatively. Furthermore, they tend to have associated lymph node metastases in 50 to 67% of cases.6,11,13 It has even been suggested that patients with primary gastrinomas of the lymph nodes most likely have an undiagnosed occult gastrinoma of the duodenum.14 In this study, we examine 63 patients who underwent resection of a sporadic primary duodenal gastrinoma for cure. We excluded patients with MEN-1 given that they tend to have multiple tumors, are difficult to cure, and have a different tumor biology.15
Each patient in our series had a primary duodenal gastrinoma located and resected. The majority were found in the first and second portions of the duodenum (53% and 31%, respectively). This finding correlates with the fact that the proximal portion of the duodenum contains the majority of G cells which are thought to be the cell of origin of most duodenal gastrinomas.16,17 Duodenal gastrinomas tend to be much smaller than pancreatic gastrinomas and are often occult (<1 cm), making them almost impossible to locate on preoperative imaging studies.7,18 US, CT, and MRI can identify up to 25%, 60%, and 20% of gastrinomas, respectively.4,19 However, the sensitivity of these imaging studies decreases with decreasing tumor size20 with tumors less than 1cm in size rarely able to be detected by CT and MRI. Only about 10% of duodenal gastrinomas are localized preoperatively. Thom et al reported that angiography, but not CT, MRI, or US diagnosed 2 of 24 duodenal gastrinomas6 and Kisker et al also reported that only 2 of 10 patients had duodenal gastrinomas localized preoperatively, both of which were larger than 1 cm.7 The size of the largest duodenal tumor in this series was less than 2 cm and the mean size was 8 mm in diameter. Consistent with the findings of others, we found that CT and MRI had little diagnostic sensitivity (6.3% and 1.8%, respectively). This indicates that the only way by which duodenal gastrinomas may be reliably localized are by intraoperative methods.6 All of the patients in this study had a duodenotomy performed for resection of the primary tumor. In 5 patients duodenotomy was the only method by which a duodenal tumor was located. External palpation proved to be better than ultrasound or transillumination locating 71% of duodenal tumors. Transillumination identified duodenal gastrinomas in 59% of the cases which is slightly lower than the 64 to 84% rate reported in other series.5,9,21 The reason for this is not entirely clear. However, the findings here support the use of duodenotomy routinely in all patients as duodenal primaries often are missed by other means including IOUS, palpation, and transillumination.
Although duodenal gastrinomas are often small, 50 to 67% of patients with duodenal primaries can have lymph node metastases found at operation.6,11,13 Our study supports this fact as 60% of patients had lymph node metastases. Eight patients were found to have lymph nodes involved with gastrinoma at a prior operation in which the duodenal primary was not localized. If left untreated, patients with gastrinoma may develop liver metastases which are associated with a significantly shorten survival of less than 30% by 10 years.22–24 Fraker et al have demonstrated that resection of all known tumor will improve survival and significantly decrease the incidence of hepatic metastases compared with patients managed medically.13
Because of the high incidence of lymph node metastases associated with duodenal gastrinomas, knowledge of their potential anatomic location in relation to the primary tumor would allow the surgeon to focus on completely dissecting all lymph nodes in that area intraoperatively. By doing this, lymph nodes with occult metastases might be resected and the cure rate might be improved. We analyzed the distribution of lymph node metastases found at the time of operation in 38 of the 63 patients in this study by mapping their location in relation to the duodenal primary. Patients who had primary duodenal tumors located above the ampulla of Vater, in general, harbored positive lymph nodes in the superior periduodenal area, celiac axis, or periportal area. Those with primary tumors in the third and fourth portions of the duodenum had positive lymph nodes located most commonly in the superior mesenteric artery or inferior periduodenal areas. Lymph nodes were found close to the primary tumor in most cases. However, resection of positive nodes was associated with a 50% relapse rate indicating that in some occult disease is being left behind at the time of operation. Prophylactic lymph node dissection in the region of the primary tumor may be useful to extirpate occult disease. Long-term cure is possible after resection of positive lymph nodes. Lymph node metastases have not been shown to adversely effect survival after resection with curative intent4 and in this series the disease-specific survival was 100% at a median 10-year follow-up. Because of the excellent survival and the fact that chemotherapy for this condition can be quite toxic,1 we do not advocate adjuvant therapy for patients with resected lymph node metastases.
In summary, primary duodenal gastrinomas tend to be small and located in the first and second portions of the duodenum. The majority of patients with duodenal ZES die of other causes and thus, tend to have indolent disease. Sixty percent of patients have associated positive lymph node metastases at the time of operation. These tend to be most commonly located close to the primary tumor. Prophylactic lymph node dissection at the time of operation may improve or allow 1 to reduce the dose of antisecretory medication. Thus, duodenal gastrinomas should be resected and improved effort should be given to identifying and resecting positive lymph nodes at the time of operation.
Footnotes
Reprints: H. Richard Alexander, MD, Surgery Branch/Center for Cancer Research, Building 10, Room 2B07, 10 Center Drive, National Cancer Institute/National Institutes of Health, Bethesda, MD 20892-1502. Richard__Alexander@nih.gov.
REFERENCES
- 1.Alexander HR Jr, Jensen RT. Pancreatic endocrine tumors. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001:1788–1813. [Google Scholar]
- 2.Metz DC, Jensen RT. Advances in gastric antisecretory therapy in Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Basel, Switzerland: S. Karger; 1995:240–257. [Google Scholar]
- 3.Hirschowitz BI, Mohnen J, Shaw S. Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrom. Aliment Pharmacol Ther. 1996;10:507–522. [DOI] [PubMed] [Google Scholar]
- 4.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison Syndrome. N Engl J Med. 1999;341:635–644. [DOI] [PubMed] [Google Scholar]
- 5.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thom AK, Norton JA, Axiotis CA, et al. Location, incidence and malignant potential of duodenal gastrinomas. Surgery. 1991;110:1086–1093. [PubMed] [Google Scholar]
- 7.Kisker O, Bastian D, Bartsch D, et al. Localization, malignant potential and surgical management of gastrinomas. World J Surg. 1998;22:651–658. [DOI] [PubMed] [Google Scholar]
- 8.Norton JA, Cromack DT, Shawker TH, Sugg SL, Norton JA, Fraker DL. Intraoperative ultrasonographic localization of islet cell tumors. Ann Surg. 1988;207:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frucht H, Norton JA, London JF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination, a prospective study. Gastroenterology. 1990;99:1622–1627. [DOI] [PubMed] [Google Scholar]
- 10.Hofman JW, Fox PS, Wilson SD. Duodenal wall tumors and the Zollinger-Ellison syndrome. Arch Surg. 1973;107:334–339. [DOI] [PubMed] [Google Scholar]
- 11.Delcore R Jr, Cheung LY, Friesen SR. Characteristics of duodenal wall gastrinomas. Am J Surg. 1990;160:621–624. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Horvath K, Udekwu A, et al. Gastrinomas: a 42-year experience. World J Surg. 1990;14:365–376. [DOI] [PubMed] [Google Scholar]
- 13.Fraker DL, Norton JA, Alexander HR, et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. Ann Surg. 1989;209:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. [DOI] [PubMed] [Google Scholar]
- 16.Solcia E, Capella C, Buffa R, et al. Endocrine cells of the gastrointestinal tract and related tumors. Pathol Annu. 1979;9:163–204. [PubMed] [Google Scholar]
- 17.Solcia E, Capella C, Buffa R, et al. Pathology of the Zollinger-Ellison syndrome. In: Fengolio LM, Wolff M, eds. Progress in Surgical Pathology. 1980;119.
- 18.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: results of a 10 year prospective study. Ann Surg. 1992;215:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1991;15:151–159. [DOI] [PubMed] [Google Scholar]
- 20.Wank SA, Doppman HL, Miller DL, et al. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:905–912. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA. Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann Oncol. 1999;10(Suppl 4):182–184. [PubMed] [Google Scholar]
- 22.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. [DOI] [PubMed] [Google Scholar]
- 23.Stabile BE, Passaro E. Benign and malignant gastrinoma. Am J Surg. 1984;149:144–150. [DOI] [PubMed] [Google Scholar]
- 24.Zollinger RM, Ellison EC, O’Dorision T, et al. Thirty years’ experience with gastrinoma. World J Surg. 1984;8:427–435. [DOI] [PubMed] [Google Scholar]