Abstract
BmrA from Bacillus subtilis is a half-size ABC (ATP-binding cassette) transporter involved in multidrug resistance. Although its supramolecular organization has been investigated after reconstitution in a lipid bilayer environment, and shows a dimeric and possibly a tetrameric form, the precise quaternary structure in a detergent-solubilized state has never been addressed. In the present study, BmrA was purified from Escherichia coli membranes using an optimized purification protocol and different detergents. Furthermore, the ATPase activity of BmrA and the quantity of bound lipids and detergent were determined, and the oligomeric state was analysed using SEC (size-exclusion chromatography) and analytical ultracentrifugation. The activity and the quaternary structure of BmrA appeared to be strongly influenced by the type and concentration of the detergent used. SEC data showed that BmrA could be purified in a functional form in 0.05 and 0.01% DDM (n-dodecyl-β-D-maltoside) and was homogeneous and monodisperse with an Rs (Stokes radius) of 5.6 nm that is compatible with a dimer structure. Sedimentation-velocity and equilibrium experiments unequivocally supported that BmrA purified in DDM is a dimer and excluded the presence of other oligomeric states. These observations, which are discussed in relation to results obtained in proteoliposomes, also constitute an important first step towards crystallographic studies of BmrA structure.
Keywords: ABC transporter (ATP-binding cassette transporter), analytical ultracentrifugation, detergent, multidrug resistance (MDR), oligomeric state, size-exclusion chromatography (SEC)
Abbreviations: 3D, three-dimensional; ABC, ATP-binding cassette; bMPDC, buoyant molecular mass of the PDC; C12E8, octa(ethylene glycol) dodecyl ether; CMC, critical micellar concentration; DDM, n-dodecyl-β-D-maltoside (dodecyl maltoside); FRET, fluorescence resonance energy transfer; MDR, multidrug resistance; NBD, nucleotide-binding domain; OG, n-octyl-β-D-glucopyranoside (octyl glucoside); OTG, n-octyl-β-D-thioglucopyranoside (octyl thioglucoside); PDC, protein–detergent complex; RS, Stokes radius; SEC, size-exclusion chromatography
INTRODUCTION
The transmembrane-protein-catalysed extrusion of noxious compounds from the cell is one of the most frequently used strategies for resistance to cytotoxic drugs in both prokaryotes and eukaryotes. These efflux proteins act as mechanical pumps that couple the transport of cytotoxic compounds out of cells to either a protonmotive force or ATP hydrolysis, the latter being the major mechanism of drug efflux in eukaryotes [1,2]. These ATP-powered transporters belong to the ABC (ATP-binding cassette) superfamily of membrane transporters, one of the largest protein families with substrate specificities ranging from sugars, amino acids and organic ions to large proteinaceous toxins [3]. Until recently, a large part of our knowledge on drug efflux pumps has come from biochemical, biophysical and genetic analyses that provide an indication of the membrane topology and of the function of these transporters. Most ABC transporters display a common domain organization with four core domains, two transmembrane domains defining the substrate-binding site, and two cytoplasmic NBDs (nucleotide-binding domains), which bind and hydrolyse ATP [4]. However, their supramolecular organization remains a controversial subject since biochemical and biophysical data reported so far for human P-glycoprotein [5–7], MRP (multi-drug resistance protein)1 [8,9], the Saccharomyces cerevisiae transporter Pdr5p [10], and for the human half-size transporter BCRP (breast cancer resistance protein) [11] described potential di- or tetra-meric assemblies as the functional unit for full-length or half-size ABC transporters respectively. Nevertheless, it is difficult to compare and analyse these results since they are highly dependent upon experimental conditions that may result in artifactual interpretation. Moreover, a full understanding of the mechanisms of these transporters and of the possible role of their supramolecular organization in the transport process ultimately requires detailed structural information. Major advances have come from the crystallization and determination of the high resolution 3D (three-dimensional) structures of four bacterial ABC transporters: three MsbA lipid A exporters in Escherichia coli [12], Vibrio cholera [13] and Salmonella typhimurium [14] and the vitamin B12 importer BtuCD [15]. These initial insights into the 3D structures are in agreement with a four domain organization but exhibit quite divergent conformations that are thought to be, especially for MsbA from E. coli, a possible consequence of crystallization conditions [16,17]. Furthermore, these crystal structures did not shed any light on the question of ABC transport proteins existing as multimers rather than monomers in membranes. Therefore the determination of additional high resolution structures with or without substrate, and in different functional states are needed, especially for ABC transporters involved in MDR (multi-drug resistance), for which no crystal structures are yet available.
BmrA, previously known as YvcC, was recently identified as a new MDR ABC half-size transporter from Bacillus subtilis, which is homologous with both LmrA from Lactococcus lactis and eukaryotic P-glycoprotein [18,19]. This transporter has the advantage of being overexpressed in E. coli [20] in high yields which is often the primary critical point for the structural determination of membrane proteins to high resolution and therefore represents a good model for studying the oligomeric state of MDR transporters. The supramolecular organization of BmrA has previously been investigated after reconstitution in a lipid bilayer environment: the 3D structure obtained at low resolution by cryoelectron microscopy suggested an organization corresponding to at least a homodimer and possibly a homotetramer [21]. More recently, time-resolved FRET (fluorescence resonance energy transfer) studies showed that BmrA exclusively forms a homodimer in proteoliposomes [22]. However, in some cases the supramolecular organization may simply reflect favourable protein–protein interactions during reconstitution rather than reflecting the true structure of the native state. Moreover, it is often difficult to establish whether oligomerization is purely structural (e.g. as a consequence of the general crowding of the membrane) or whether it is essential for the function of the protein. In a previous report, BmrA extracted from membranes in 1% DDM (n-dodecyl-β-D-maltoside) and subsequently purified in 0.05% DDM was shown to interact with many P-glycoprotein effectors and thus to possibly conserve a ‘native-like’ conformation throughout the purification procedure [19]. On the other hand, the existence of a monomer–dimer equilibrium during the reconstitution into proteoliposomes (i.e. using high concentrations of DDM) was recently suggested for BmrA [22]. Apart from these studies, the issue of homogeneity and the influence of detergents on the oligomerization state of BmrA have not been addressed, although these are very important parameters for structural studies of BmrA. Indeed, conflicting data currently exist in the literature concerning the oligomeric organization of some membrane proteins, as revealed by their high resolution crystal structures, that are not in agreement with results obtained using biochemical or other biophysical techniques. This is the case, amongst others, for the mitochondrial ADP/ATP carrier [23], the protein-conducting channel SecY from Methanococcus jannaschii [24] and the MDR efflux transporter EmrE from E. coli [25,26]. Although the natural supramolecular state of proteins often persists within the crystal packing structure, the compulsory use of detergents during purification and crystallization of membrane proteins, and therefore their excessive delipidation, may disrupt protein oligomers and lead to the crystallization of a non-functional and non-physiological form.
Accordingly, our objectives were to make a detailed characterization of BmrA in detergent solutions that are commonly used for structural and functional studies and to determine whether the type of detergent and its concentration modulates the activity and the oligomeric state of BmrA. We have assessed the specific ATPase activity of BmrA in the presence of non-ionic detergents, and investigated the protein's oligomeric state using nondenaturing PAGE, SEC (size-exclusion chromatography) and analytical ultracentrifugation. To the best of our knowledge, this is the first comprehensive report of different experimental approaches to determine the supramolecular organization of an ABC transporter in detergent.
EXPERIMENTAL
Chemicals
The following detergents were used: DDM, OG (n-octyl-β-D-glucopyranoside) and C12E8 [octa(ethylene glycol) dodecyl ether] from Anatrace, Hecameg® and OTG (n-octyl-β-D-thioglucopyranoside) were from Alexis Biochemicals. [14C]DDM (55 mCi/mmol) was synthesized at the Commisariat à l'énergie Atomique, Saclay, France [27]. High and low molecular mass protein standards were purchased from Amersham Biosciences (Pharmacia) and Bio-Rad.
Purification of His-tagged BmrA in DDM
Cloning and overexpression of BmrA in the C41-DE3 E. coli mutant strain were performed according to the method of Steinfels et al. [20], and solubilization of BmrA from plasma membranes was performed with 1% (w/v) DDM as described previously [19].
Thereafter, the purification protocol was optimized so as to produce BmrA suitable for crystallographic studies. All purification steps were performed at 15 °C and all buffers were filtered and degassed. The samples were centrifuged before use. BmrA was first purified by metal-chelate affinity chromatography using an FPLC system (Amersham Pharmacia). After the addition of 1 mM PMSF, solubilized membrane proteins (approx. 2 mg/ml) were loaded at a flow rate of 0.3 ml/min on to a 1 ml Ni2+-High-Trap Chelating column, pre-equilibrated with loading buffer A [50 mM potassium phosphate (pH 8), 100 mM NaCl, 15% (v/v) glycerol, 20 mM imidazole, 5 mM 2-mercaptoethanol and 0.05 or 0.01% (w/v) DDM]. After washing with 10 ml of loading buffer, the column was washed again at 0.4 ml/min with 10 ml of buffer containing 90 mM imidazole followed by an elution step using 5 ml of buffer containing 240 mM imidazole. Fractions (0.5 ml) containing BmrA were collected and dialysed for 24 h against buffer B [50 mM Hepes/KOH (pH 8), 100 mM NaCl, 10% (v/v) glycerol, 5 mM β-mercaptoethanol, 0.05 or 0.01% (w/v) DDM and 1 mM EDTA]. Finally, BmrA was purified by SEC/FPLC on a Superdex 200 10/300 GL column (Amersham) equilibrated with 2 column vol. of buffer B. A 1 ml aliquot of the protein sample (1–3 mg/ml) was loaded on to the column at a flow rate of 0.4 ml/min. In order to measure the amount of detergent bound to BmrA, buffer B was replaced by buffer C [50 mM Hepes/KOH (pH 7.35), 100 mM NaCl, 10% (v/v) glycerol, 5 mM 2-mercaptoethanol, 0.05% (w/v) DDM and 1 mM EDTA]. Fractions containing BmrA (0.5 ml) were collected, pooled and either used immediately or frozen in liquid nitrogen and stored at −80 °C.
SDS/PAGE
PAGE under denaturing conditions [0.1% (w/v) SDS] was carried out using 12% polyacrylamide separating gels according to the method of Laemmli [28]. Before loading on to a 3.6% polyacrylamide stacking gel, samples were mixed with Laemmli buffer and incubated at room temperature for 20 min. SDS/PAGE was carried out at constant voltage (200 V) for 45 min and stained with Coomassie Brilliant Blue R250.
Non-denaturing PAGE
Non-denaturing electrophoresis was performed on a 1.5 mm thick, 8% Tris/glycine polyacrylamide gel. Protein samples (approx. 1–10 μg) were mixed with a half vol. of sample buffer [60 mM Tris (pH 8.8), 25% (v/v) glycerol, 1% Coomassie Brillant Blue R250 and 0.05% (w/v) DDM] and loaded on to a large-pore (3.6% polyacrylamide) stacking gel.
Detergent [0.05% (w/v) DDM] was added to the 30% acrylamide/bisacrylamide solution before polymerization was initiated by ammonium persulphate and TEMED (N,N,N′,N′-tetramethylethylenediamine). Electrophoresis was carried out at constant voltage (100 V in the stacking gel and 200 V in the separating gel) at room temperature using running buffer [50 mM Tris (pH 8.3), 200 mM glycine and 0.05% (w/v) DDM], and gels were stained with Coomassie Brillant Blue R250.
Determination of BmrA concentration
The concentration of BmrA was routinely determined by a modified Lowry assay (Pierce) which includes a precipitation step to avoid any interference owing to detergents, lipids or other buffer components [29]. Based on the molar absorption coefficient (ϵ280 BmrA=36.3 mM−1·cm−1), a correction factor of 1.17 was used to estimate protein concentration from the Lowry assay.
Rs (Stokes radius) determination by SEC/FPLC or SEC-HPLC
Protein standards with an RS known to be unchanged in the presence of DDM were used to calibrate gel-filtration columns in the same detergent as used for analysis of BmrA samples [30,31]. Partition coefficients (KD) of protein standards and the BmrA–detergent complex were determined by:
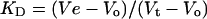 |
where the elution volume (Ve) is the position of the peak in millilitres. Blue Dextran and tryptophan-methyl-ester were used as markers of the void volume (Vo) and the total excluded volume (Vt) respectively. The calibration curve representing the logarithm of RS as a function of KD was used to determine the RS of BmrA.
Determination on a Superdex 200 10/300 GL column
Thyroglobulin (radius 8.6 nm; molecular mass 669±100.3 kDa), ferritin (6.3 nm, 440±66 kDa), catalase (5.2 nm, 232±34.8 kDa), aldolase (4.6 nm, 158±23.7 kDa), BSA (3.5 nm, 67±6.7 kD), ovalbumin (2.8 nm, 43±6.5 kDa), chymotrypsinogen (2.1 nm, 25±6.3) and ribonuclease A (1.75 nm, 13.7±2.06 kDa) were used to calibrate the Superdex 200 10/300 GL column used for BmrA purification. The markers were solubilized in buffer B. The calibration standard curve (Log RS=−1.35 KD+1.04) was used to interpolate the RS of the PDC (protein–detergent complex), with a correlation coefficient R2=0.99.
Determination on a TSK G3000SWXL column
Thyroglobulin, ferritin, γ-globulin (5.2 nm, 158±23.7 kDa), ovalbumin, myoglobin (1.9 nm, 17±2.6 kDa) and vitamin B12 (0.9 nm, 1.35±0.2 kDa) were used to calibrate the TSK G3000SWXL column used for the determination of the amount of detergent bound to the protein. The markers were solubilized in buffer C. The calibration standard curve (Log RS=−86 KD+0.93) was used to interpolate RS of the BmrA PDC with a correlation coefficient R2=0.93.
Evaluation of the amount of bound detergent
Detergent-binding to BmrA was determined using analytical SEC-HPLC in the presence of [14C]DDM. Fractions containing BmrA eluted after the second purification step were concentrated by ultrafiltration on a Centricon 100 (Amicon Ultra, Millipore) column to a final volume of 200 μl, and loaded at 1 ml/min on to a TSK-gel™ G3000SWXL column equilibrated with 2 vol. of the following buffer C* [50 mM Hepes/KOH (pH 7.35), 100 mM NaCl, 5% (v/v) glycerol and 0.05% (w/v) [14C]DDM approx. 1.75 μCi/ml]. The fractions containing BmrA were pooled, reconcentrated on a 30 kDa Centricon (Ultrafree Biomax, Millipore) column to a final volume of 100 μl and re-run on the same column to ensure complete exchange of DDM for [14C]DDM. Aliquots (25 μl), from the baseline, and fractions collected along the elution profile, were counted for radioactivity (c.p.m.bl and c.p.m.peak respectively). An accurate determination of the protein concentration in the eluted fractions was performed as described above.
The amount of bound detergent δD, (g of DDM/g of protein) was calculated using the equation:
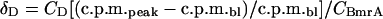 |
where CD and CBmrA correspond to the concentration of the detergent in the buffer and the concentration of protein in the selected fraction respectively [32].
Lipid analysis
The presence of lipids in the purified samples was determined by analytical TLC. Purified BmrA was spotted on to a silica gel plate and dissolved in chloroform/methanol/25% aqueous ammonia (65:25:5, by vol.). Lipids and detergents were first visualized under UV light and the plates were finally stained with iodide (Sigma) vapour in a sealed chromatography tank. The total amount of phospholipid co-purified with BmrA was determined using samples extracted directly from the purified protein, employing a phosphorus assay derived from Chen et al. [33]. Lipids were extracted from the purified protein with 3 vol. of chloroform/methanol (2:1). The organic phase containing lipids was dried in a Speed vac and redissolved in 100 μl of H2O and 250 μl of 70% HClO4. The samples were thereafter placed in borosilicate-glass tubes and heated to 220 °C over a sand bath for 1 h. After cooling, a solution composed of 800 μl of H2O, 400 μl of ammonium molybdate 1.25%, and 400 μl of 4-methyl-amino-phenol sulphate was added to the tubes. The tubes were heated in a water bath at 100 °C for 5 min and subsequently cooled to room temperature, and the absorbance was read at 797 nm against the reference standards in a Beckman DU 520 spectrophotometer. A typical standard curve based on six samples containing between 0 and 75 nmoles of KH2PO4, gave a straight line (R2>0.96). The lipid concentration of the unknown samples was determined as the average of three measurements. The lipid:protein mass ratio, δL, was calculated for the lipids using the molecular mass of egg yolk phosphatidylcholine.
Detergent exchange
In order to assess the monodispersity, as well as the stability of BmrA in different detergents, DDM was exchanged for OG, C12E8, Hecameg® or OTG using affinity chromatography during the first purification step. The High-Trap chelating column was first washed with 10 ml of buffer A, and thereafter the gel was equilibrated with 15 column vol. of buffer A′ [same composition as in buffer A but replacing DDM with OG, C12E8, Hecameg® or OTG at a concentration 4-fold higher than their CMC (critical micellar concentration)]. Further elution, dialysis and gel-filtration steps were conducted as described in the purification protocol, replacing DDM in all buffers with the appropriate detergent.
Assay of ATPase activity
ATPase activity was routinely measured at 37 °C in 50 mM Hepes/KOH, pH 8, in the presence of an ATP-regenerating system coupled to NADH oxidation (0.3 mM NADH, 4 mM phosphoenol pyruvate, 60 μg/ml pyruvate kinase and 32 μg/ml lactate dehydrogenase) as previously described [34]. Detergent (DDM or C12E8 at the same concentration as in the samples), 10 mM MgCl2 and ATP were added to the experimental buffer. The reaction was initiated by the addition of samples containing 1–15 μg of protein, and NADH oxidation was recorded at A340 for several minutes. The activity was expressed as units/mg of protein (1 unit=1 μmol ATP hydrolysed/min). Alternatively, ATPase activity was measured using the method of Taussky and Shorr [35], and inhibition of the reaction by vanadate was assayed, this procedure was employed in order to avoid any interference from the enzymes used in the previous ATPase assay. Briefly, the purified protein (10 μg) was incubated at 37 °C in 50 mM Hepes/KOH (pH 8), 10 mM ATP, 10 mM MgCl2, 0.05 or 0.01% DDM and a regenerating ATP system (5 mM phosphoenolpyruvate and 50 μg/ml pyruvate kinase) in a final volume of 2.5 ml. The amount of Pi liberated was then determined by a colorimetric assay (740 nm), using NaH2PO4 as a standard. Aliquots (500 μl) of solution were withdrawn at four different times and the reaction was stopped by denaturing the protein with 500 μl of 10% SDS. Taussky and Shorr reagent (500 μl) [35] was then added, and the absorbance was read at 740 nm after 15 min of colour development at room temperature. Data were fitted to give a straight line of which the slope corresponds to the amount of Pi released per min (R2>0.97) and the protein activity can be calculated by dividing the amount of Pi released per min by the amount of protein equivalent to 500 μl of solution withdrawn.
ATPase activity was hence expressed in μmol of Pi liberated/min per mg of protein. To detect the effect of vanadate, the same procedure was followed, using a final concentration of 500 μM Vi in the standard curve and the ATPase assay.
Analytical ultracentrifugation experiments
Sedimentation-equilibrium and velocity experiments were carried out in a Beckman XL-I analytical ultracentrifuge using an AN-60 TI rotor (Beckman instruments), at 20 °C. After purification samples were handled immediately in the elution buffer used during the SEC (buffer B) containing either 0.05% (w/v) DDM, 0.01% (w/v) DDM or 0.02% (w/v) C12E8, or were handled after precise dilution in appropriate buffers. The density (ρ) and viscosity (η) of the solutions [which contained 10% (v/v) glycerol] were measured as 1.036±0.001 g/ml and 1.46±0.01 cP, at 20 °C on a DMA 5000 Anton Paar density meter and an AMVn Anton Paar viscosity meter respectively. The BmrA molecular mass (MP) and partial specific volume (νP) were estimated from the amino-acid composition to be 66254 kDa and 0.752 ml/g respectively, using Sednterp software (http://www.bbri.org/RASMB/rasmb.html). The partial specific volumes were considered for the detergents: νD, 0.824 ml/g for DDM and 0.97 ml/g for C12E8 [32]; for the lipids νL, 0.981 ml/g for egg yolk phosphatidylcholine [36]; for water νW, 1.00 ml/g.
Sedimentation-velocity experiments
Sedimentation-velocity experiments were carried out at 42000 g, using 100 or 400 μl of the protein samples loaded into the 2-channel 0.3 or 1.2 cm path-length centerpieces respectively. Absorbance scans were recorded overnight at 276 nm with a 0.003 cm radial-step size, at 5 min intervals. Sedimentation-velocity profiles were analysed using size distribution analysis from the Sedfit program (version 8.9 developed by P. Schuck; http://www.analyticalultracentrifugation.com) providing a continuous distribution of apparent sedimentation coefficients, c(s) [37]. Typically 20 regularly-spaced experimental profiles obtained from a total of 6 h sedimentation were globally modelled. The continuous distribution of apparent sedimentation coefficient [c(s)] analysis was performed with 200 particles on a grid of 300 radial points, calculated with a frictional ratio, f/f0, of 1.25 (corresponding to globular species), for sedimentation coefficients in the range of 2–12 S (in DDM) and 2–50 S (in C12E8). The partial specific volume for the PDC (νPDC) was considered to be 0.8 ml/g in DDM and 0.9 ml/g in C12E8 (an intermediate value between the values of the protein and of the detergent). For the regularization procedure, which allows a c(s) for a regular solution to be obtained, a confidence level of 0.7 was used.
Sedimentation-equilibrium experiments
Sedimentation-equilibrium experiments were performed at 7000 g for 4 days, followed by an accelaration step at 42000 g using 40 or 160 μl of the BmrA samples loaded on to 10 or 40 μl FC-43 oil in the 2-channel centerpieces of 3 or 12 mm path-lengths respectively, against 60 or 200 μl of solvent as references. Radial scans of the absorbance at 276 nm were taken every 3 h. The equilibrium condition was checked using Winmatch V7 software, the equilibrium files were prepared using Reedit v9 and the analysis performed globally using Winnonlin v1.060 software, assuming one ideal species, and thus allowing the determination of the buoyant molecular mass of the complex (bMPDC). These programs are available at (http://www.rasmb.bbri.org/rasmb/windows/uconn_uaf/). Baseline and bMPDC values were similar regardless of the experimental baselines used (determined either experimentally at 42000 g or from the fitted curves).
RESULTS
Characterization of BmrA as purified in 0.05% DDM: optimization of the BmrA purification protocol in 0.05% DDM
The aim of this study was to design a rapid and reproducible purification protocol leading to a pure, homogenous, monodisperse, stable and active sample, which furthermore would be suitable for crystallization studies.
In the first step, BmrA fused to a C-terminal 6-His tag, was solubilized in 1% DDM from E. coli plasma membranes according to the protocol described by Steinfels et al. [20]. The previous purification protocol [18,19] was thereafter adapted although retaining the composition of buffers: pH 8, 0.05% (w/v) DDM and 15% (v/v) glycerol. Indeed, pH 8 is optimal for BmrA activity (C. Orelle, A. Di Pietro and J.-M. Jault, unpublished work) and glycerol has been shown to stabilize many purified proteins probably by reducing the activity of the water molecules and hence minimizing denaturizing effects [38].
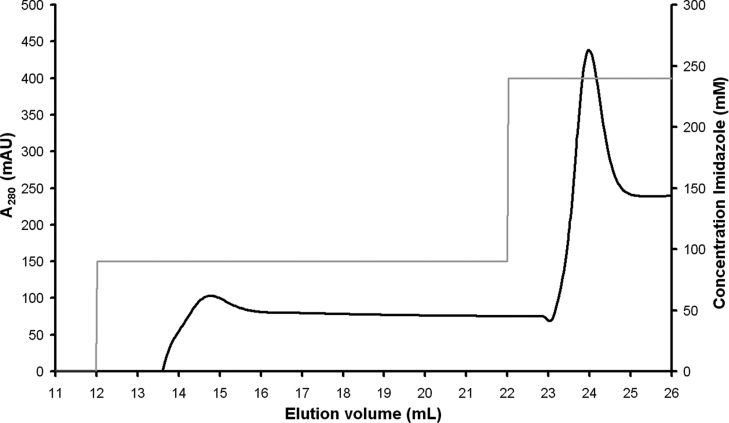
BmrA was first purified by affinity chromatography on an FPLC system instead of employing the batch method with Ni-NTA (nickel–nitrilotriacetic acid) agarose, as used previously. In 0.05% DDM, the protein was retained on the column at pH 8.0 in buffer A and thereafter selectively eluted by two successive steps in 90 and 240 mM imidazole. The first step eluted the majority of the contaminants, whereas BmrA was eluted as a sharp peak during the second step at a protein concentration range of 1–3 mg/ml (Figure 1). Protein in this peak was approx. 80% pure, as estimated by Coomassie Blue staining after SDS/PAGE (Figure 2A). Only fractions containing the purest and most concentrated BmrA were collected.
Figure 1. Elution of DDM-solubilized BmrA protein from an Ni2+-High Trap chelating column.
The absorbance of the protein was monitored at 280 nm, and BmrA eluted in 240 mM imidazole. AU, absorbance units.
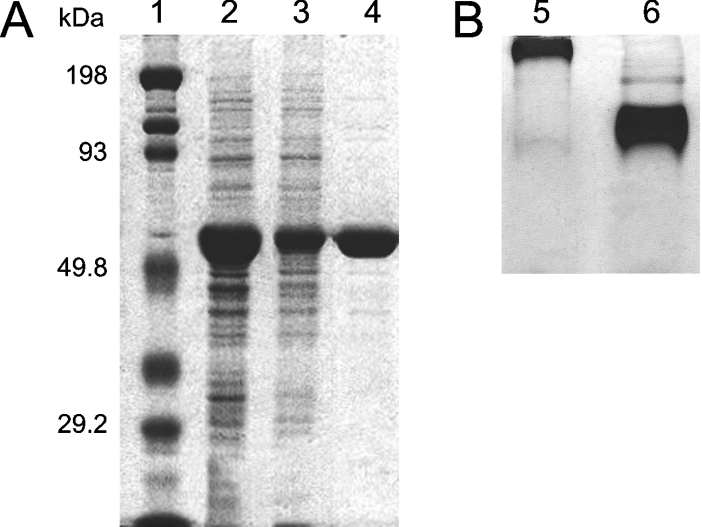
Figure 2. Purification of BmrA as analysed by PAGE.
(A) SDS/PAGE. Lane 1, molecular mass markers; protein standards in kDa are indicated on the left side. Lane 2, protein eluted from the Ni2+-High Trap chelating column during the 240 mM imidazole step. Lane 3, protein eluted by SEC/FPLC on a Superdex 200 10/300 GL column at 8.9 ml. Lane 4, protein eluted by SEC/FPLC on a Superdex 200 10/300 GL column at 11.5 ml. (B) Non-denaturing PAGE of fractions eluted by SEC/FPLC on a Superdex 200 10/300 GL column. Lane 5, BmrA eluted at 8.9 ml. Lane 6, BmrA eluted at 11.5 ml.
Thereafter, collected fractions were dialysed against buffer B which allowed simultaneous removal of imidazole, and 1 mM EDTA was added which decreased aggregation of the protein (results not shown).
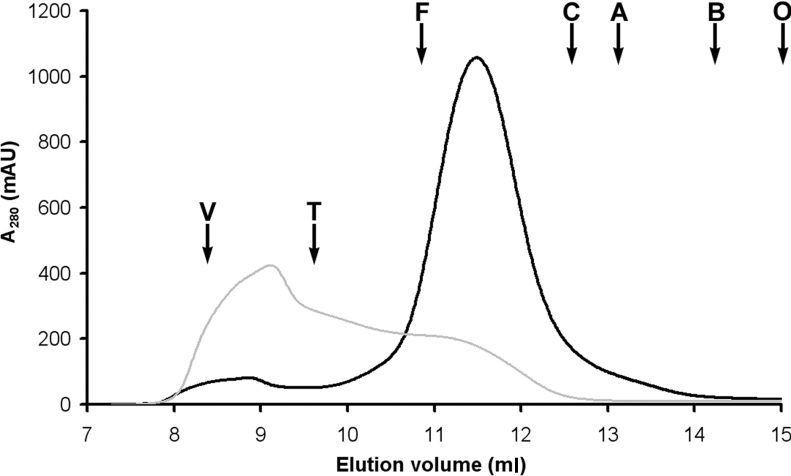
In order to improve protein purity an additional SEC/FPLC purification step was added. The sample (after concentration to 1 ml) was loaded on to a Superdex 200 10/300 GL column. With 0.05% DDM in the eluent, the initial BmrA–PDC eluate shows a very minor peak at approx. 8.9 ml (near the void volume) and a sharp, symmetrical peak at an elution volume of approx. 11.5 ml at 280 nm (Figure 3). Both peaks contain BmrA as shown by SDS/PAGE (Figure 2A). Nondenaturing PAGE containing micellar concentrations of DDM suggest good apparent homogeneity of DDM solubilized BmrA in the major peak and the presence of large BmrA aggregates in the minor peak (Figure 2B). This last result was confirmed by sedimentation-velocity experiments (results not shown). The elution profiles were very reproducible despite varying protein concentrations. Moreover, the purity of protein in the major peak was improved as compared with the initial purification protocol, where it was estimated to be approx. 95% (Figure 2A). Unfortunately, with this final purification step, approx. 10% of the protein was lost. Thereafter, the collected fractions, corresponding to the major peak (concentration range 0.5–1.5 mg/ml), were frozen in liquid nitrogen and kept at −80 °C.
Figure 3. SEC of BmrA in 0.05% DDM (black line) and in 0.02% C12E8 (grey line).
Conditions are described in the Experimental section. Typical elution profiles (A280) are shown with the positions of calibration markers: T, thyroglobulin; F, ferritin; C, catalase; A, aldolase; B, bovine serum albumin; O, ovalbumin. The void volume is indicated by V. AU, absorbance units.
Activity of purified BmrA
It is well known that detergents can affect protein integrity and therefore one of the crucial aims of this study was to obtain BmrA in an active form after purification in 0.05% DDM. Direct measurement of the transport activity of BmrA is not feasible in a detergent solution owing to the vectorial nature of the transport process. The activity of BmrA purified in 0.05% DDM was thus assessed using the two ATPase activity assays described in the Experimental section. ATP was hydrolysed at a rate of 1.2±0.3 μmol/min per mg of protein at 37 °C, and the addition of vanadate (500 μM) resulted in approx. 80% inhibition, ruling out the possibility of ATPase activity owing to a contaminant. It can therefore be concluded that the protein remains active after solubilization and purification in this detergent, suggesting at least a homodimer organization, which is reportedly the functional form of the protein [19,22].
RS of the PDC in 0.05% DDM
Knowledge of the PDC composition is essential for protein crystallization studies. Hence it was necessary to determine the oligomeric state of BmrA in the BmrA solution eluted from the column, and to measure DDM and bound-lipid content of the protein. RS and the apparent molecular mass of the BmrA–DDM–lipid micelles in this preparation were analysed by SEC. The BmrA–DDM RS was determined to be 5.6±0.4 nm using either a Superdex 200 10/300 GL column during the gel-filtration purification step on the FPLC or a TSK-gel™ G3000SWXL column on a HPLC system during the measurement of detergent binding to BmrA. The pH in buffer C (7.35 instead of 8.0 pH in buffer B) was chosen according to the pH stability of the column, but did not affect the oligomeric state of BmrA. Although it is not rigorous to calibrate a gel filtration in terms of molecular mass [36], this elution position corresponds to an apparent molecular mass of 300±30 kDa for the PDC.
Detergent binding to BmrA
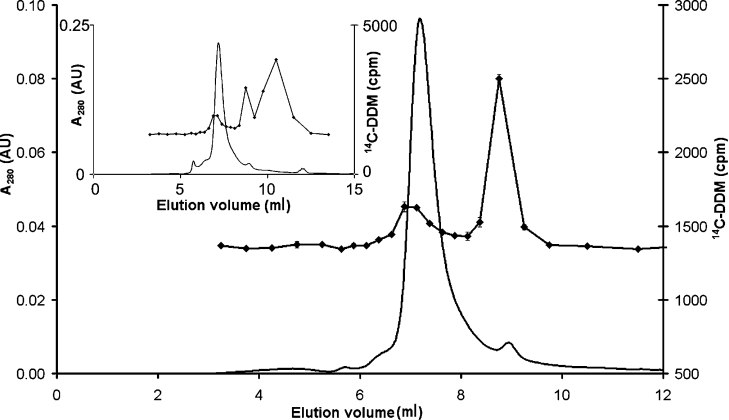
Two successive gel-filtration steps in buffer C* were performed on a TSK-gel™ G3000SWXL column to remove excess micelles and ensure complete exchange of DDM for [14C]DDM. The absorbance profile from the second chromatography step and the detergent's elution profile are shown in Figure 4. The protein peak was associated with a rise in detergent concentration above the baseline, corresponding to protein-bound DDM. The second peak represents the free-detergent micelles. Indeed after rechromatography, most of the free detergent that was in excess was removed and the amount of DDM bound to BmrA (δD) was calculated as 1.5±0.6 g/g.
Figure 4. SEC elution profile at 280 nm (−) and [14C]DDM-binding level (◆) after the second run of BmrA on a TSK-gel G3000SWXL column.
Inset, elution profile at 280 nm (straight line) absorbance and the [14C]DDM-binding level (◆) after the first run. AU, absorbance units.
Phospholipids bound to BmrA
Analytical TLC confirmed the presence of phospholipids that were co-purified with BmrA (results not shown). Measurements from purified BmrA, using a phosphorus assay, indicated the co-purification of approx. δL=0.07:1 (w/w), assuming one phosphate per phospholipid, and thus indicating that a small number of lipids are tightly associated with BmrA.
Hydrodynamic properties of the BmrA–DDM complex
Considering a 66 kDa molecular mass for tagged BmrA, the estimated amount of bound detergent and lipids is 99 and 4.6 kDa per polypeptide respectively, and thus the molecular mass for the monomer complex is approx. 170 kDa. The apparent molecular mass from gel filtration data, 300±30 kDa, is hence consistent with BmrA existing as a globular dimer. If the experimental values of δD, δL and RS are used for a dimeric form of BmrA, then the f/f0 ratio is calculated to be 1.2, which is within the range 1.2–1.3 commonly found for globular species (see Supplementary eqns 6 and 7 http://www.BiochemJ.org/bj/395/bj3950345add.htm). In order to interpret the data for a monomer, the f/f0 ratio should be 1.5, which is rather high and would correspond to a very asymmetric monomer. A tetramer is unlikely, since f/f0 would be below the minimal theoretical value of 1, corresponding to a non-hydrated sphere.
In order to determine the hydrodynamic properties of BmrA purified in 0.05% DDM, a combination of sedimentation-equilibrium and -velocity analyses using analytical ultracentrifugation was performed.
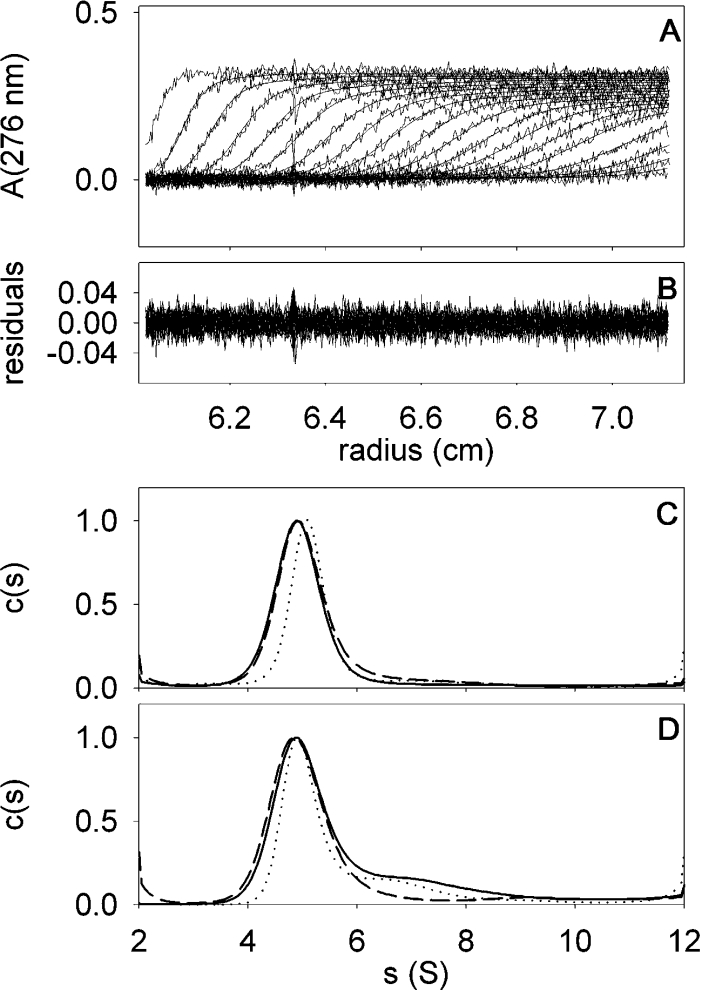
Sedimentation-velocity experiments were performed at various BmrA concentrations and analysed in terms of the distribution of sedimentation coefficients, allowing qualitative evaluation of protein homogeneity and self-association capacity. The distribution obtained for the BmrA peak eluted at 11.5 ml in the SEC step, showed a rather symmetrical peak (Figure 5C). The same pattern is observed for samples from two different protein preparations: samples at 0.13 and 0.5 mg/ml obtained without a dialysis step in EDTA, as well as at 1.2 mg/ml obtained with a dialysis step before SEC. These results indicate the reproducibility of the purification protocol and that no significant concentration dependency exists. Moreover, the protein sample appears to be homogeneous in 0.05% DDM and no self-association equilibrium process seems to occur.
Figure 5. Sedimentation-velocity of BmrA in the presence of 0.05 and 0.01% DDM.
Panel A, superimposed experimental sedimentation profiles and the fitted curves from the c(s) analysis (using Sedfit) of BmrA at 0.5 mg/ml in the presence of 0.05% DDM. The last profile corresponds to 6 h sedimentation at 42000 g and 20 °C. The statistical noise has been subtracted. Panel B, superimposed differences between the experimental and fitted curves from Panel A. Panel C, c(s) analysis of BmrA, in the presence of 0.05% DDM at 0.13, 0.5 and 1.2 mg/ml (continuous, dotted and dashed lines respectively). Panel D, c(s) analysis of BmrA, with 0.01% DDM at 0.3, 0.9 and 0.24 mg/ml (continuous, dotted and dashed lines respectively), the latter sample was diluted before the experiments from a sample at 0.05% DDM, and the two others were from SEC. A detailed description is given in the Experimental section.
For this peak, a mean value of s=5.05 S (s20,0w=8.9±0.3 S) was calculated. Combining the value of s with the measured Stokes radius, RS=5.6±0.4 nm from gel filtration, leads to a bMPDC (buoyant molecular mass of the PDC) of 46.9±1.9 kDa (see Supplementary eqn 3 at http://www.BiochemJ.org/bj/395/bj3950345add.htm). Considering the values of 1.5 and 0.07 g/g for δD and δL respectively, this corresponds to an MP (molecular mass of the protein) of 110±20 kDa (see Supplementary eqn 4 at http://www.BiochemJ.org/bj/395/bj3950345add.htm) and is close to values calculated for the dimer, bMPDC=56.8±11.3 kDa and MP=132.5 kDa, as compared with the monomer or any other supramolecular form (Table 1).
Table 1. BmrA structural parameters as deduced from SEC, phospholipid assays and analytical ultracentrifugation.
| Detergent | |||
|---|---|---|---|
| Structural data | Unit | DDM (0.05/0.01%) | C12E8 (0.02%) |
| SEC/FPLC and HPLC | RS (nm) (1) | 5.6±0.4 | ∼5.6† |
| δD (g/g) (2) | 1.5±0.6 | 1.5† | |
| Phospholipid assay | δL (g/g) (3) | 0.07±0.02 | 0.07† |
| Sedimentation velocity data | s (S) (4) | 5.05 | 3.2 |
| Combined data (1), (2), (3) and (4) | bMPDC (kDa) | 46.9±1.9 | 29.7±1.4 |
| MPDC (kDa) | 314±15 | 420±20 | |
| MP (kDa) | 110±20 | 146±20 | |
| Sedimentation equilibrium data | bMPDC (kDa) (5) | 50±4.5 | |
| Combined data (2), (3) and (5) | MPDC (kDa) | 334±30 | |
| MP (kDa) | 117±20 | ||
| Combined data (2), (3), (4) and (5) | f/f° | 1.18±0.08 | 1.14±0.08 |
| Combined data (2), (3)* and (6) | VPDC (ml/g) | 0.821±0.001 | 0.897±0.016 |
| bMPDC, dimer (kDa) | 56.8±11.3 | 26.7±0.4 | |
| MPDC (kDa) | 340±80 | 340±80 | |
| Protein sequence (6) | MP (kDa) | 66.254 | 66.254 |
| VP (ml/g) | 0.752 | 0.752 | |
* With a hydration of 0.3 g/g, and considering in the second column the values for δD and δL obtained in the presence of DDM.
† Assumed value based on value obtained with DDM.
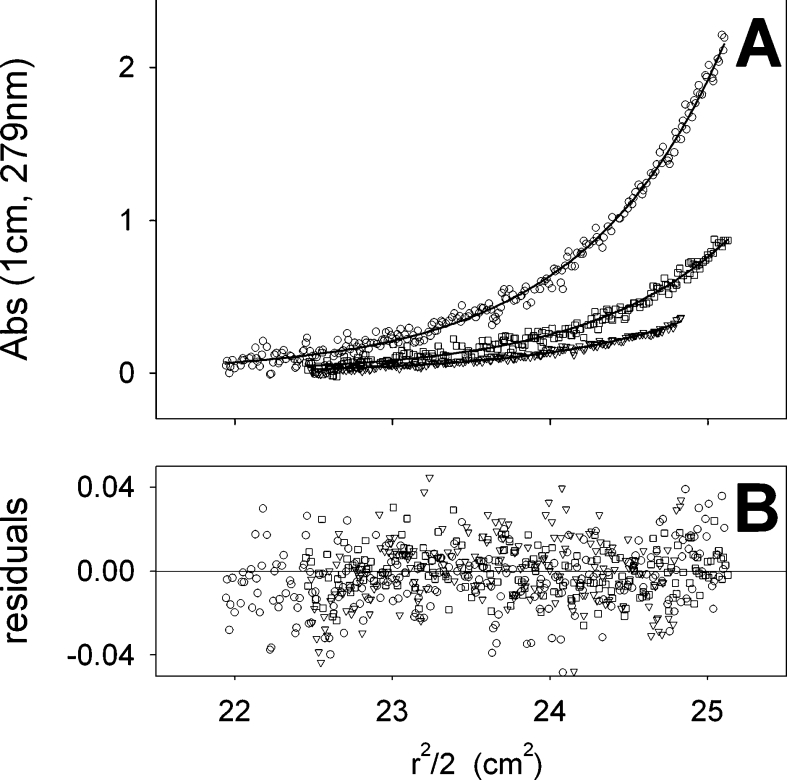
For sedimentation-equilibrium experiments, three concentrations of BmrA purified in 0.05% DDM were used. The experimental equilibrium sedimentation profiles obtained for the three protein concentrations could be nicely fitted according to a one-species model, as shown in Figure 6. A bMPDC of 50 kDa with uncertainties of 3 and 6 kDa, was obtained when considering either experimentally determined or fitted baselines respectively, and resulted in an apparent molecular mass of 117±20 kDa for BmrA (see Supplementary eqns 4 and 5 at http://www.BiochemJ.org/bj/395/bj3950345add.htm), a value that is consistent with BmrA as a dimer (Table 1). This is in agreement with the sedimentation-velocity experiments and suggests that BmrA exists as a single and homogeneous dimeric species in 0.05% DDM.
Figure 6. Sedimentation-equilibrium of BmrA in the presence of 0.05% DDM.
Equilibrium sedimentation profiles of BmrA at 1.2 mg/ml (circle), 0.6 mg/ml (square) and 0.24 mg/ml (triangle) were obtained after centrifugation at 7000 g for four days at 20 °C. Panel A, experimental data (symbols) and the result of their fitting using the model of one ideal non-interacting species (upper curve, circles; middle curve, squares; lower curve, triangles). Panel B, differences between the experimental and the fitted curves. The determined buoyant molecular mass was 50 kDa.
Influence of the detergent
Four other detergents were examined in order to study the influence of the nature and concentration of the detergent on monodispersity, activity and stability of BmrA. These detergents are associated with different chemical properties; long versus short acyl-chain lengths, low and high CMCs.
DDM [0.05% (w/v)] was exchanged with 2.6% (w/v) Hecameg®, 0.8% (w/v) OG, 1.1% (w/v) OTG or 0.02% (w/v) C12E8 respectively, during the affinity chromatography step in the FPLC, and similar profiles were obtained, but with lower purification yields (results not shown). For OG and OTG, all of the protein precipitated during the dialysis step. In Hecameg®, a major portion precipitated during dialysis and a remaining soluble portion eluted at 14 ml during the gel filtration (results not shown), thus indicating the probable dissociation of the protein dimer into monomers.
With 0.02% C12E8 in the eluent, the initial BmrA PDC eluted by SEC/FPLC showed a large asymmetrical peak eluting between 8.5 and 12.5 ml, therefore indicating a high amount of aggregation (Figure 3). Sedimentation-velocity experiments clearly confirmed that the sample was heterogeneous, as can be seen in Figure 7 for a fraction eluted between 11 and 12 ml (elution volume corresponding to the PDC in DDM, i.e. RS approx. 5.6 nm), which sediments with s20,0w between approx. 7 and 30 S. In 0.02% C12E8, the smallest species, s=3.2 S (s20,0w=7 S), is present in different chromatographic fractions. Assuming the same composition (e.g. quantity of bound lipids, δL, and detergent, δD) as those measured in DDM, the resulting bMPDC corresponds to a dimer (Table 1).
Figure 7. Sedimentation-velocity of BmrA at 0.25 mg/ml in the presence of 0.02% C12E8.
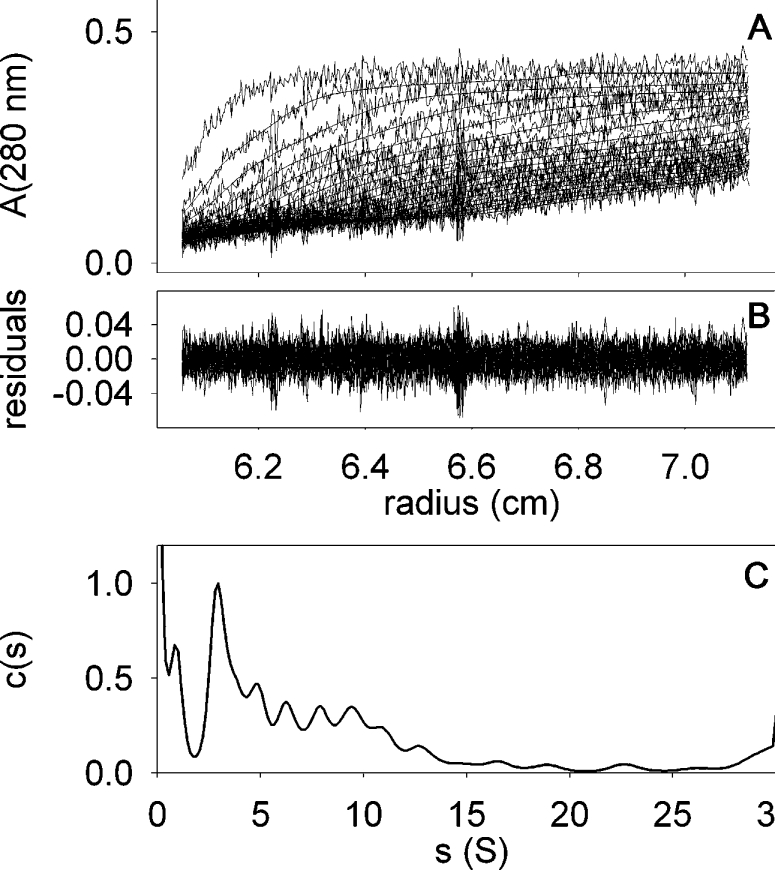
Panel A, superimposed experimental sedimentation profiles and those fitted from c(s) analysis using Sedfit. The last profile corresponds to 6 h of sedimentation at 42000 g and 20 °C. The statistical noise was subtracted. Panel B, superimposed differences between the experimental and modelled profiles. Panel C, c(s) analysis.
The ATPase activity measurement of this same fraction gives a maximal rate of 0.5 μmol/min per mg of protein (3-fold lower than in 0.05% DDM). Since the concentrations used for both detergents are well over their CMC (4–5-fold), this suggests either a partial denaturation of the protein caused by this detergent or that C12E8 has an inhibitory effect on the ATPase activity of BmrA.
With the aim of minimizing the formation of detergent micelles during the subsequent concentration of the protein, the state of the protein in DDM concentrations lowered to the CMC were also examined.
In 0.01% DDM, the characteristics of purified BmrA were similar to those found in 0.05% DDM, but with a slightly lower yield. The retention time and the elution profile upon SEC/FPLC were virtually identical to those seen in 0.05% DDM (results not shown). Moreover, the sedimentation coefficient was the same as that measured in the presence of 0.05% DDM by sedimentation-velocity experiments and is independent of the protein concentration (Figure 5, Panel D). An additional contribution at s=7 S (s20,0w=13 S) is seen, corresponding to larger species. Nevertheless, for a freshly diluted sample from 0.05 to 0.01% DDM, this phenomenon was not observed, (Figure 5; broken line, Panel D), suggesting a slow aggregation process. In 0.01% DDM, the ATPase activity was higher than in 0.05% DDM, with a rate of approx. 2.2±0.2 μmol ATP hydrolysed/min per mg of protein and was highly sensitive to vanadate, since the addition of 500 μM vanadate decreased ATPase activity by 90%.
DISCUSSION
We have characterized the oligomeric state of detergent-solubilized BmrA, purified from E. coli membranes with an optimized protocol, using non-denaturing PAGE, SEC and analytical ultracentrifugation.
Protein arising from the previously established purification protocol [18,19] was not pure enough for crystallographic studies, and the complete characterization of the detergent-purified protein, including the determination of detergent and lipid concentrations was lacking, factors that are otherwise known to cause irreproducibility and failure in sample preparation for structural analysis. We therefore optimized the purification protocol before crystallization studies. The use of 0.05% DDM for purification was conserved from the previous protocol, whereas the use of FPLC and the addition of an SEC step have allowed us to design a more rapid and reproducible method which also improves the purity of the protein. This new preparation resulted in a homogeneous and active sample that was eluted as a single peak upon SEC, suggesting that BmrA exists in a single oligomeric state in 0.05 and 0.01% DDM, namely a dimer. However, the nature of this dimer (dimerization or not of the NBDs) remains uncertain.
As demonstrated for other membrane proteins [26,39–43], the activity and the quaternary structure of BmrA has been shown to be strongly affected by the type of detergent used. Studies of the oligomeric status of BmrA were extended to include the effect of non-denaturing detergents upon the exchange with DDM present in the first purification step. In each detergent, BmrA exhibited different self-association behaviour. Our data indicate that shorter chain (C6–C8) hydrocarbon detergents significantly inactivate the protein by either leading to the formation of aggregates or by disrupting the functional dimers. SEC and sedimentation-velocity experiments clearly showed highly polydisperse BmrA in the presence of C12E8, which in turn fails to keep the protein in an active and stable state.
The molecular mass of the purified BmrA–detergent complex in solutions containing DDM has been determined using SEC and analytical ultracentrifugation, and titration of bound detergent and lipids. The measured value is consistent with that calculated for a dimer, assuming an entity composed of two copies of BmrA, and approx. 12 lipids and approx. 380 DDM molecules, largely exceeding the aggregation number of DDM (approx. 110, see [32]). The δD value of bound detergent/gram of protein (measured here as 1.5) is within the range found for other membrane proteins [32,44]. The quantity of lipids that co-migrate with BmrA is among the smallest values found as compared with other membrane proteins purified in detergent for which such an analysis has been carried out, but remains entirely consistent with those calculated for samples prepared by more than one chromatographic step [32,39,40,42,43]. Although few phospholipids were present, the protein remains monodisperse and stable in DDM.
The physical authenticity of the experimentally determined RS of the BmrA–DDM dimer can be checked by comparison with a BmrA 3D model [45], based on the crystal structure of the homologous protein MsbA from Vibrio cholerae [13] for the transmembrane regions, and on the crystal structure of the MJ0796 ATP-binding cassette for the cytoplasmic domains [46]. It can be assumed that the 12-transmembrane α-helices form a compact transmembrane segment with a diameter of approx. 5 nm and that DDM molecules extend 3 nm perpendicular to the transmembrane segment of BmrA resulting in a radius of approx. 6 nm [32]. An estimate of the height of BmrA perpendicular to the membrane surface indicates a radius of 6 nm which is also based on the 3D model of BmrA; this approximation of the molecular dimensions of the dimeric BmrA–DDM complex is consistent with the experimentally determined RS of 5.6±0.4 nm.
The results obtained in the presence of DDM and presented here are consistent with the determination of the supramolecular organization of BmrA in a lipid bilayer environment using FRET [22], although they exclude a possible tetrameric association as suggested from the low-resolution structure of BmrA obtained by electron microscopy [21]. Furthermore, our results exclude the existence of a monomer–dimer equilibrium in micellar DDM solutions at concentrations below 0.05%. However, we cannot rule out the possibility that higher oligomers such as tetramers could exist in the membranes, as well as monomers in other detergents or at higher DDM concentrations. It is possible that tetramers in the membrane dissociate into dimers upon solubilization, whereas monomer–monomer interactions within a dimer appear much stronger and dimer–dimer interactions if any, must be relatively weak and thus less important for correct functioning of the protein.
Our data show that BmrA retains significant ATPase activity and is almost completely inhibited by vanadate after purification in 0.05 or 0.01% DDM, demonstrating that it remains folded in an active conformation, which has been established to be a dimer in the present study. Although this activity is higher than that reported for the majority of ABC transporters [47–51], it appears to be lower than when the protein is reconstituted into proteoliposomes, with a value of 6.5 μmol of ATP hydrolysed/min per mg of protein [18,19]. This is clearly not due to the presence of monomeric or other non-cooperative forms of BmrA in the sample, as illustrated in the present study, but rather that ATPase assays with detergent-solubilized and purified preparations often result in an underestimation of the true potential basal activity because of the strong influence of the lipid environment, as has been shown for P-glycoprotein [52].
The results obtained in the present study clearly indicate that the choice of detergent is crucial in order to obtain pure, homogeneous and monodisperse BmrA samples. In order to carry out crystallization experiments, a highly purified, monodisperse and stable protein sample is required. DDM used at concentrations between 0.01 and 0.05% was shown to be the most reliable detergent for purification and crystallization studies by maintaining homodimeric BmrA with a high degree of monodispersity and activity, which theoretically reflects the physiological arrangement of this transporter.
Also the use of DDM at 0.01% appeared to be an interesting alternative to the initial concentration of 0.05%, since BmrA activity, as well as its monodispersity was maintained as shown by SEC/FPLC and analytical ultracentrifugation.
Online data
Acknowledgments
The present work was supported by CNRS (Centre National de la Recherche Scientifique) including the interdisciplinary program GDR2478 – Membrane Proteins and colloidal assemblies. We thank Samuel Tranier for technical help and advice, and Xavier Robert for help with the Figures.
References
- 1.Putman M., van Veen H. W., Konings W. N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000;64:672–693. doi: 10.1128/mmbr.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges-Walmsley M. I., McKeegan K. S., Walmsley A. R. Structure and function of efflux pumps that confer resistance to drugs. Biochem. J. 2003;376:313–338. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dassa E., Bouige P. The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 4.Higgins C. F. ABC transporters: physiology, structure and mechanism–an overview. Res. Microbiol. 2001;152:205–210. doi: 10.1016/s0923-2508(01)01193-7. [DOI] [PubMed] [Google Scholar]
- 5.Poruchynsky M. S., Ling V. Detection of oligomeric and monomeric forms of P-glycoprotein in multidrug resistant cells. Biochemistry. 1994;33:4163–4174. doi: 10.1021/bi00180a009. [DOI] [PubMed] [Google Scholar]
- 6.Boscoboinik D., Debanne M. T., Stafford A. R., Jung C. Y., Gupta R. S., Epand R. M. Dimerization of the P-glycoprotein in membranes. Biochim. Biophys. Acta. 1990;1027:225–228. doi: 10.1016/0005-2736(90)90311-b. [DOI] [PubMed] [Google Scholar]
- 7.Jette L., Potier M., Beliveau R. P-glycoprotein is a dimer in the kidney and brain capillary membranes: effect of cyclosporin A and SDZ-PSC 833. Biochemistry. 1997;36:13929–13937. doi: 10.1021/bi970737+. [DOI] [PubMed] [Google Scholar]
- 8.Soszynski M., Kaluzna A., Rychlik B., Sokal A., Bartosz G. Radiation inactivation suggests that human multidrug resistance-associated protein 1 occurs as a dimer in the human erythrocyte membrane. Arch. Biochim. Biophys. 1998;354:311–316. doi: 10.1006/abbi.1998.0687. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg M. F., Mao Q., Holzenburg A., Ford R. C., Deeley R. G., Cole S. P. The structure of the multidrug resistance protein 1 (MRP1/ABCC1) crystallization and single-particle analysis. J. Biol. Chem. 2001;276:16076–16082. doi: 10.1074/jbc.M100176200. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira-Pereira A., Marco S., Decottignies A., Nader J., Goffeau A., Rigaud J. L. Three-dimensional reconstruction of the Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J. Biol. Chem. 2003;278:11995–11999. doi: 10.1074/jbc.M212198200. [DOI] [PubMed] [Google Scholar]
- 11.Xu J., Liu Y., Yang Y., Bates S., Zhang J. T. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 12.Chang G., Roth C. B. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science (Washington DC) 2001;293:1793–1800. doi: 10.1126/science.293.5536.1793. [DOI] [PubMed] [Google Scholar]
- 13.Chang G. Structure of MsbA from Vibrio cholera: a multidrug resistance ABC transporter homolog in a closed conformation. J. Mol. Biol. 2003;330:419–430. doi: 10.1016/s0022-2836(03)00587-4. [DOI] [PubMed] [Google Scholar]
- 14.Reyes C. L., Chang G. Structure of the ABC transporter MsbA in complex with ADP vanadate and lipopolysaccharide. Science (Washington DC) 2005;308:1028–1031. doi: 10.1126/science.1107733. [DOI] [PubMed] [Google Scholar]
- 15.Locher K. P., Lee A. T., Rees D. C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science (Washington DC) 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 16.Campbell J. D., Biggin P. C., Baaden M., Sansom M. S. Extending the structure of an ABC transporter to atomic resolution: modeling and simulation studies of MsbA. Biochemistry. 2003;42:3666–3673. doi: 10.1021/bi027337t. [DOI] [PubMed] [Google Scholar]
- 17.Buchaklian A. H., Funk A. L., Klug C. S. Resting state conformation of the MsbA homodimer as studied by site-directed spin labeling. Biochemistry. 2004;43:8600–8606. doi: 10.1021/bi0497751. [DOI] [PubMed] [Google Scholar]
- 18.Orelle C., Dalmas O., Gros P., Di Pietro A., Jault J. M. The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA. J. Biol. Chem. 2003;278:47002–4708. doi: 10.1074/jbc.M308268200. [DOI] [PubMed] [Google Scholar]
- 19.Steinfels E., Orelle C., Fantino J. R., Dalmas O., Rigaud J. L., Denizot F., Di Pietro A., Jault J. M. Characterization of YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis. Biochemistry. 2004;43:7491–7502. doi: 10.1021/bi0362018. [DOI] [PubMed] [Google Scholar]
- 20.Steinfels E., Orelle C., Dalmas O., Penin F., Miroux B., Di Pietro A., Jault J. M. Highly efficient over-production in E. coli of YvcC, a multidrug-like ATP-binding cassette transporter from Bacillus subtilis. Biochim. Biophys. Acta. 2002;1565:1–5. doi: 10.1016/s0005-2736(02)00515-1. [DOI] [PubMed] [Google Scholar]
- 21.Chami M., Steinfels E., Orelle C., Jault J. M., Di Pietro A., Rigaud J. L., Marco S. Three-dimensional structure by cryo-electron microscopy of YvcC, an homodimeric ATP-binding cassette transporter from Bacillus subtilis. J. Mol. Biol. 2002;315:1075–1085. doi: 10.1006/jmbi.2001.5309. [DOI] [PubMed] [Google Scholar]
- 22.Dalmas O., Do Cao M. A., Lugo M. R., Sharom F. J., Di Pietro A., Jault J. M. Time-resolved fluorescence resonance energy transfer shows that the bacterial multidrug ABC half-transporter BmrA functions as a homodimer. Biochemistry. 2005;44:4312–4321. doi: 10.1021/bi0482809. [DOI] [PubMed] [Google Scholar]
- 23.Pebay-Peyroula E., Dahout-Gonzalez C., Kahn R., Trezeguet V., Lauquin G. J., Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature (London) 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berg B., Clemons W. M., Jr, Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. X-ray structure of a protein-conducting channel. Nature (London) 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 25.Ma C., Chang G. Structure of the multidrug resistance efflux transporter EmrE from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2852–2857. doi: 10.1073/pnas.0400137101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Winstone T. L., Jidenko M., Maire M., Ebel C., Duncalf K. A., Turner R. J. Organic solvent extracted EmrE solubilized in dodecyl maltoside is monomeric and binds drug ligand. Biochem. Biophys. Res. Commun. 2005;327:437–445. doi: 10.1016/j.bbrc.2004.11.164. [DOI] [PubMed] [Google Scholar]
- 27.Kragh-Hansen U., le Maire M., Noel J. P., Gulik-Krzywicki T., Møller J. V. Transitional steps in the solubilization of protein-containing membranes and liposomes by nonionic detergent. Biochemistry. 1993;32:1648–1656. doi: 10.1021/bi00057a032. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal. Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 30.Le Maire M., Aggerbeck L. P., Monteilhet C., Andersen J. P., Møller J. V. The use of high-performance liquid chromatography for the determination of size and molecular weight of proteins: a caution and a list of membrane proteins suitable as standards. Anal. Biochem. 1986;154:525–535. doi: 10.1016/0003-2697(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 31.Klammt C., Lohr F., Schafer B., Haase W., Dotsch V., Ruterjans H., Glaubitz C., Bernhard F. High level cell-free expression and specific labeling of integral membrane proteins. Eur. J. Biochem. 2004;271:568–580. doi: 10.1111/j.1432-1033.2003.03959.x. [DOI] [PubMed] [Google Scholar]
- 32.Møller J. V., le Maire M. Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J. Biol. Chem. 1993;268:18659–18672. [PubMed] [Google Scholar]
- 33.Chen P. S., Toribara T. Y., Warner H. Microdetermination of phosphorus. Anal. Chem. 1956;28:1756–1758. [Google Scholar]
- 34.Jault J. M., Di Pietro A., Falson P., Gautheron D. C. Alteration of apparent negative cooperativity of ATPase activity by alpha-subunit glutamine 173 mutation in yeast mitochondrial F1. Correlation with impaired nucleotide interaction at a regulatory site. J. Biol. Chem. 1991;266:8073–8078. [PubMed] [Google Scholar]
- 35.Taussky H. H., Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- 36.Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974;13:2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- 37.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutcher M. P. Maintaining protein stability. In: Deutcher M.P., editor. Methods in Enzymology. San Diego, U.S.A.: Academic Press; 1990. pp. 83–89. [DOI] [PubMed] [Google Scholar]
- 39.Breyton C., Tribet C., Olive J., Dubacq J. P., Popot J. L. Dimer to monomer conversion of the cytochrome b6 f complex. Causes and consequences. J. Biol. Chem. 1997;272:21892–21900. doi: 10.1074/jbc.272.35.21892. [DOI] [PubMed] [Google Scholar]
- 40.Musatov A., Ortega-Lopez J., Robinson N. C. Detergent-solubilized bovine cytochrome c oxidase: dimerization depends on the amphiphilic environment. Biochemistry. 2000;39:12996–3004. doi: 10.1021/bi000884z. [DOI] [PubMed] [Google Scholar]
- 41.Josse D., Ebel C., Stroebel D., Fontaine A., Borges F., Echalier A., Baud D., Renault F., Le Maire M., Chabrieres E., Masson P. Oligomeric states of the detergent-solubilized human serum paraoxonase (PON1) J. Biol. Chem. 2002;277:33386–33397. doi: 10.1074/jbc.M200108200. [DOI] [PubMed] [Google Scholar]
- 42.Lemieux M. J., Reithmeier R. A., Wang D. N. Importance of detergent and phospholipid in the crystallization of the human erythrocyte anion-exchanger membrane domain. J. Struct. Biol. 2002;137:322–332. doi: 10.1016/s1047-8477(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 43.Butler P. J., Ubarretxena-Belandia I., Warne T., Tate C. G. The Escherichia coli multidrug transporter EmrE is a dimer in the detergent-solubilised state. J. Mol. Biol. 2004;340:797–808. doi: 10.1016/j.jmb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Friesen R. H., Knol J., Poolman B. Quaternary structure of the lactose transport protein of Streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem. 2000;275:33527–33535. doi: 10.1074/jbc.M004066200. [DOI] [PubMed] [Google Scholar]
- 45.Dalmas O., Orelle C., Foucher A. E., Geourjon C., Crouzy S., Di Pietro A., Jault J. M. The Q-loop disengages from the first intracellular loop during the catalytic cycle of the multidrug ABC transporter BmrA. J. Biol. Chem. 2005;280:36857–36864. doi: 10.1074/jbc.M503266200. [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y. R., Blecker S., Martsinkevich O., Millen L., Thomas P. J., Hunt J. F. The crystal structure of the MJ0796 ATP-binding cassette. Implications for the structural consequences of ATP hydrolysis in the active site of an ABC transporter. J. Biol.Chem. 2001;276:32313–32321. doi: 10.1074/jbc.M100758200. [DOI] [PubMed] [Google Scholar]
- 47.Davidson A. L., Laghaeian S. S., Mannering D. E. The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J. Biol. Chem. 1996;271:4858–4863. [PubMed] [Google Scholar]
- 48.Liu C. E., Liu P. Q., Ames G. F. Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter) J. Biol. Chem. 1997;272:21883–21891. doi: 10.1074/jbc.272.35.21883. [DOI] [PubMed] [Google Scholar]
- 49.Chang X. B., Hou Y. X., Riordan J. R. ATPase activity of purified multidrug resistance-associated protein. J. Biol. Chem. 1997;272:30962–30968. doi: 10.1074/jbc.272.49.30962. [DOI] [PubMed] [Google Scholar]
- 50.Vigano C., Grimard V., Margolles A., Goormaghtigh E., van Veen H. W., Konings W. N., Ruysschaert J. M. A new experimental approach to detect long-range conformational changes transmitted between the membrane and cytosolic domains of LmrA, a bacterial multidrug transporter. FEBS Lett. 2002;530:197–203. doi: 10.1016/s0014-5793(02)03485-3. [DOI] [PubMed] [Google Scholar]
- 51.Doerrler W. T., Raetz C. R. ATPase activity of the MsbA lipid flippase of Escherichia coli. J. Biol. Chem. 2002;277:36697–36705. doi: 10.1074/jbc.M205857200. [DOI] [PubMed] [Google Scholar]
- 52.Al-Shawi M. K., Polar M. K., Omote H., Figler R. A. Transition state analysis of the coupling of drug transport to ATP hydrolysis by P-glycoprotein. J. Biol. Chem. 2003;278:52629–52640. doi: 10.1074/jbc.M308175200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.