Abstract
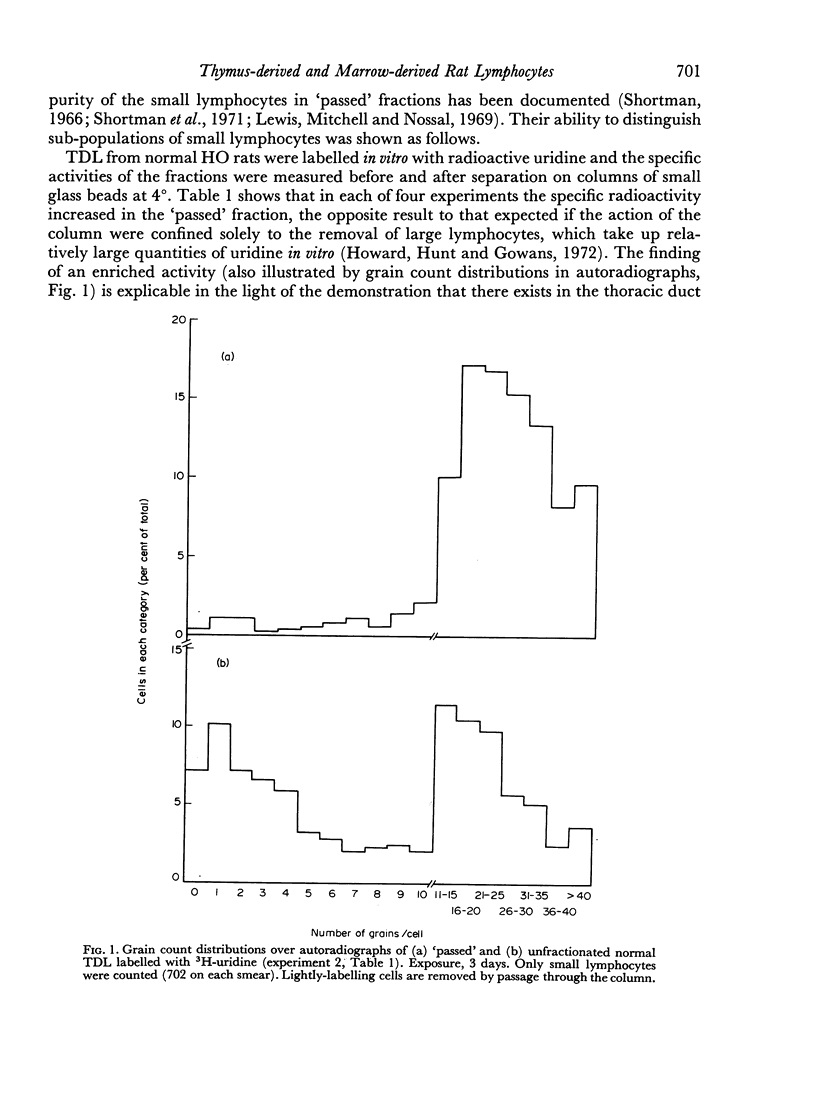
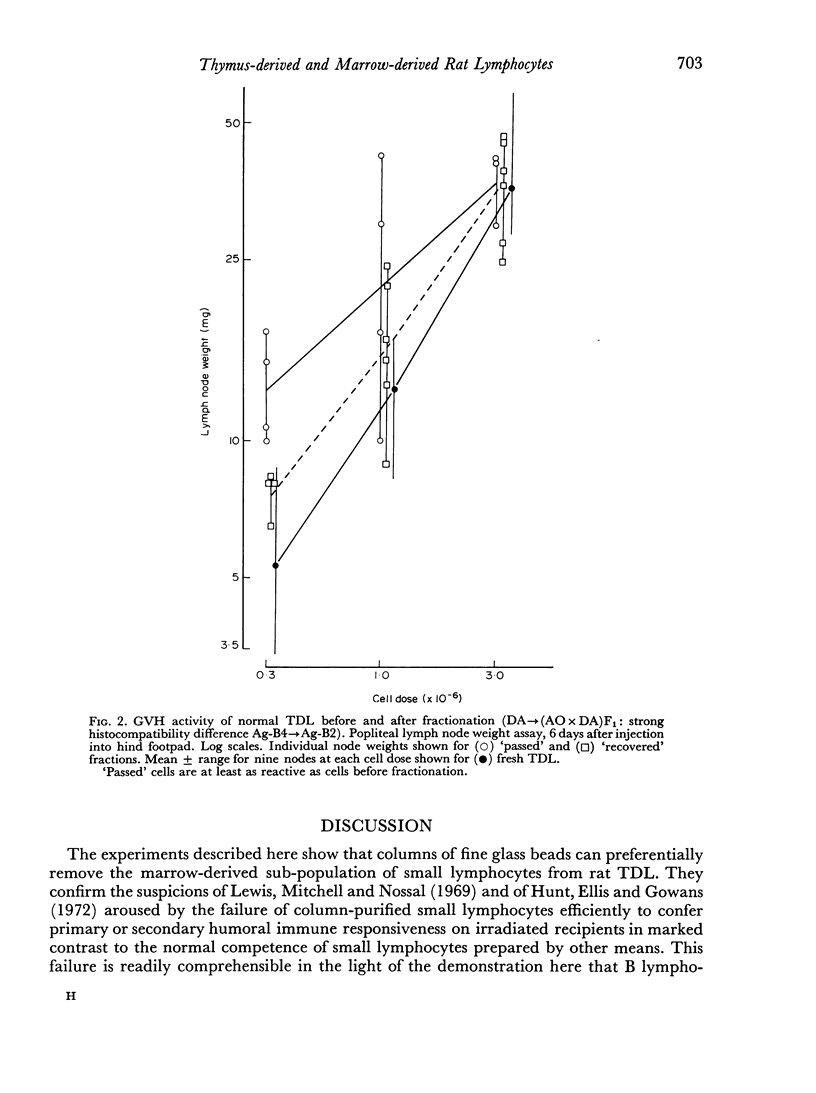
Rat thoracic duct lymphocytes were passed through columns of fine siliconed glass beads according to the method of Shortman (1966). On the basis of the degree of labelling with uridine in vitro and of surface antigenic markers it was shown that marrow-derived small lymphocytes were preferentially retained on the columns, causing the concentration of thymus-derived small lymphocytes in the filtered cells. Filtered cells retained normal graft-versus-host (GVH) activity. The proportion of thymus-derived small lymphocytes in normal rat lymph was estimated at a maximum of 63–87 per cent. Previous reports on the immunocompetence of filtered cells may readily be explained by these findings.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H. The effects of anti-theta antiserum upon graft-versus-host activity of spleen and lymph node cells. Cell Immunol. 1972 Mar;3(3):461–469. doi: 10.1016/0008-8749(72)90251-1. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. I. Rapid method for isolation of theta-positive antigen-stimulated cells. Immunology. 1972 Jun;22(6):959–965. [PMC free article] [PubMed] [Google Scholar]
- Howard J. C., Hunt S. V., Gowans J. L. Identification of marrow-derived and thymus-derived small lymphocytes in the lymphoid tissue and thoracic duct lymph of normal rats. J Exp Med. 1972 Feb 1;135(2):200–219. doi: 10.1084/jem.135.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C. The life-span and recirculation of marrow-derived small lymphocytes from the rat thoracic duct. J Exp Med. 1972 Feb 1;135(2):185–199. doi: 10.1084/jem.135.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. V., Ellis S. T., Gowans J. L. The role of lymphocytes in antibody formation. IV. Carriage of immunological memory by lymphocyte fractions separated by velocity sedimentation and on glass bead columns. Proc R Soc Lond B Biol Sci. 1972 Sep 19;182(1067):211–231. doi: 10.1098/rspb.1972.0076. [DOI] [PubMed] [Google Scholar]
- Lewis H., Mitchell J., Nossal G. J. Subpopulations of rat and mouse thoracic duct small lymphocytes in the Salmonella flagellar antigen system. Immunology. 1969 Dec;17(6):955–967. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Thymus-derived cells in mouse thoracic duct lymph. Nat New Biol. 1971 Apr 28;230(17):267–270. doi: 10.1038/newbio230267a0. [DOI] [PubMed] [Google Scholar]
- Plotz P. H., Talal N. Fractionation of splenic antibody-forming cells on glass bead columns. J Immunol. 1967 Dec;99(6):1236–1242. [PubMed] [Google Scholar]
- Rosenstreich D. L., Shevach E., Green I., Rosenthal A. S. The uropod-bearing lymphocyte of the guinea pig. Evidence for thymic origin. J Exp Med. 1972 May 1;135(5):1037–1048. doi: 10.1084/jem.135.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Davie J. M., Rosenstreich D. L., Blake J. T. Depletion of antibody-forming cells and their precursors from complex lymphoid cell populations. J Immunol. 1972 Jan;108(1):279–281. [PubMed] [Google Scholar]
- Salerno A., Pontieri G. M. Heterogeneity of populations of antibody-forming cells fractionated by glass bead columns. Clin Exp Immunol. 1969 Jul;5(1):209–212. [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Howard J. C. Collaboration between thymus-derived and marrow-derived thoracic duct lymphocytes in the hemolysin response of the rat. Cell Immunol. 1972 Mar;3(3):430–441. doi: 10.1016/0008-8749(72)90248-1. [DOI] [PubMed] [Google Scholar]
- Shortman K., Szenberg A. The size and density distribution of fowl blood lymphocytes initiating a graft-versus-host reaction. Aust J Exp Biol Med Sci. 1969 Feb;47(1):1–9. doi: 10.1038/icb.1969.1. [DOI] [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. 1. The use of glass bead columns to separate small lymphocytes, remove damaged cells and fractionate cell suspensions. Aust J Exp Biol Med Sci. 1966 Jun;44(3):271–286. [PubMed] [Google Scholar]
- Shortman K., Williams N., Jackson H., Russell P., Byrt P., Diener E. The separation of different cell classes from lymphoid organs. IV. The separation of lymphocytes from phagocytes on glass bead columns, and its effect on subpopulations of lymphocytes and antibody-forming cells. J Cell Biol. 1971 Mar;48(3):566–579. doi: 10.1083/jcb.48.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]


