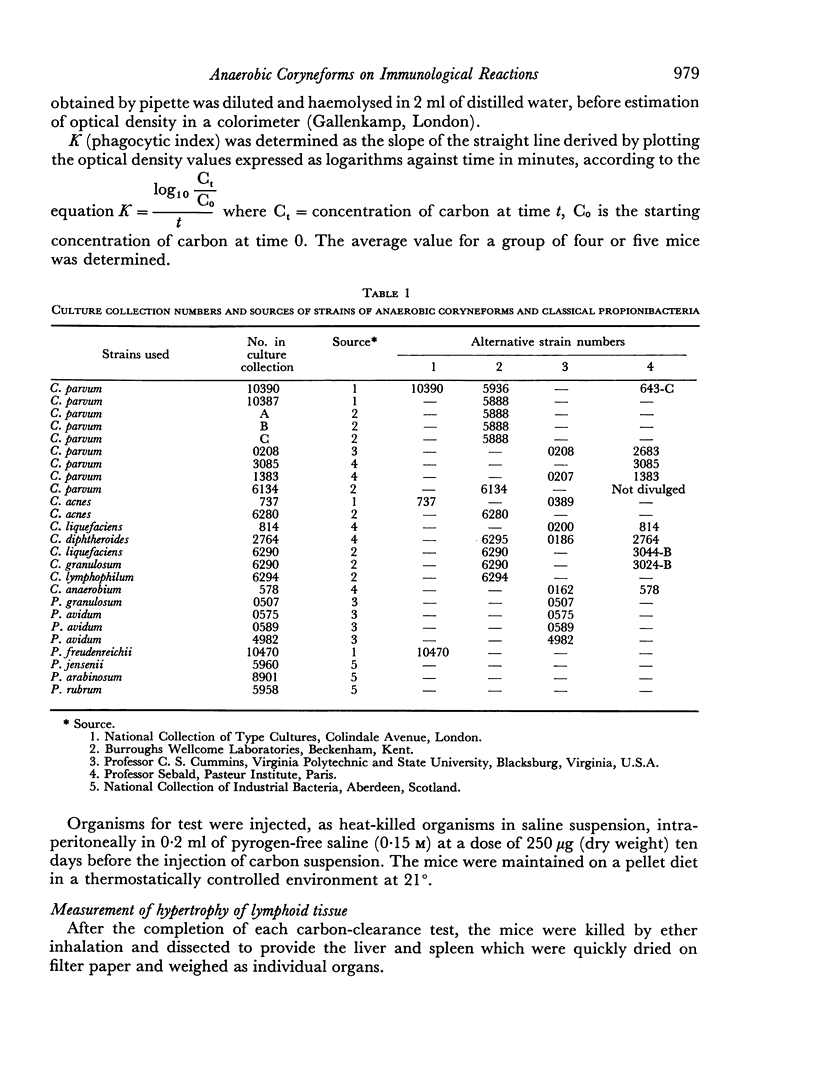
Abstract
A wide range of different strains of anaerobic coryneforms and classical propionibacteria have been surveyed for some of their macrocytostimulant effects (included under this term are the ability to increase the rate of phagocytic uptake of carbon after intravenous injection of the latter into mice, ability to stimulate an increase in lysosomal hydrolases and ability to exert directly a chemotactic stimulus on macrophages) and their ability to increase humoral and cellular immunity when admixed with an immunogen.
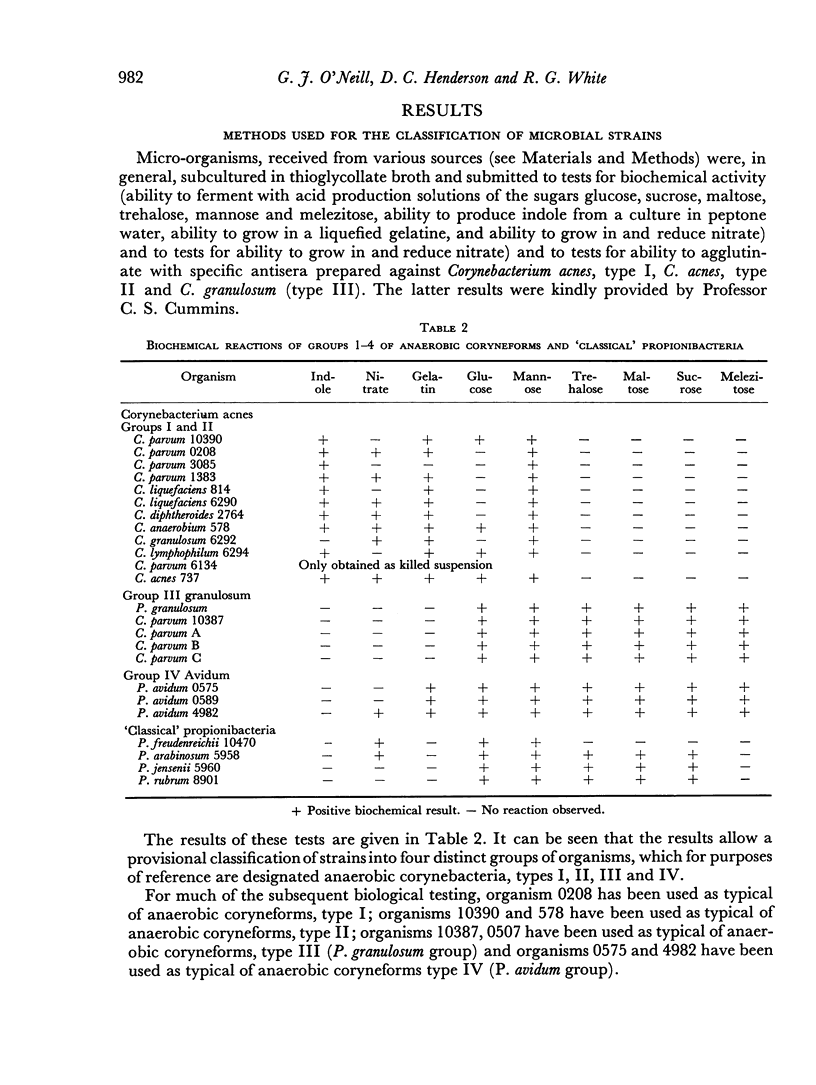
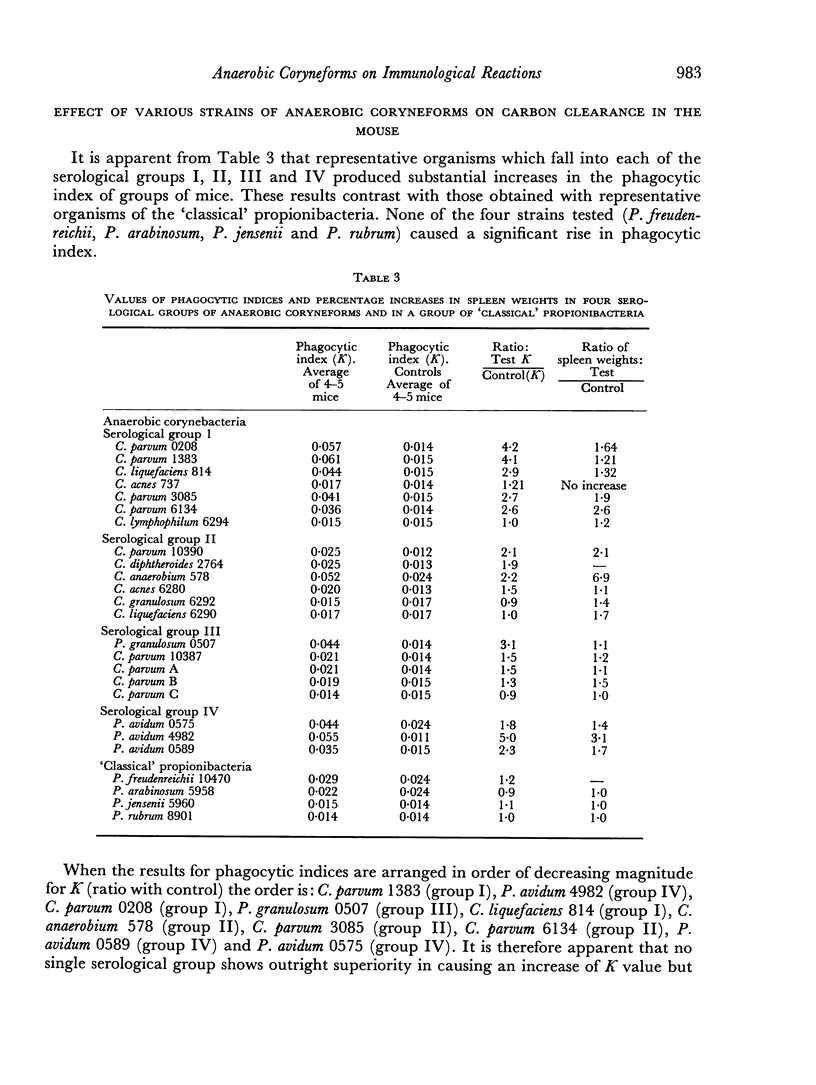
Of twenty-one strains of anaerobic coryneforms tested, fifteen strains were able to produce an increase of the phagocytic index in mice of at least 50 per cent. Micro-organisms that were effective in these tests occurred in each of the four main serological groups. Although no single serological group showed outright superiority in causing an increase of K, three of the five strains which had highest activity belonged to serological group I. Four organisms representing the `classical' propionibacteria were also tested, but none of these caused an increase of phagocytic index in mice.
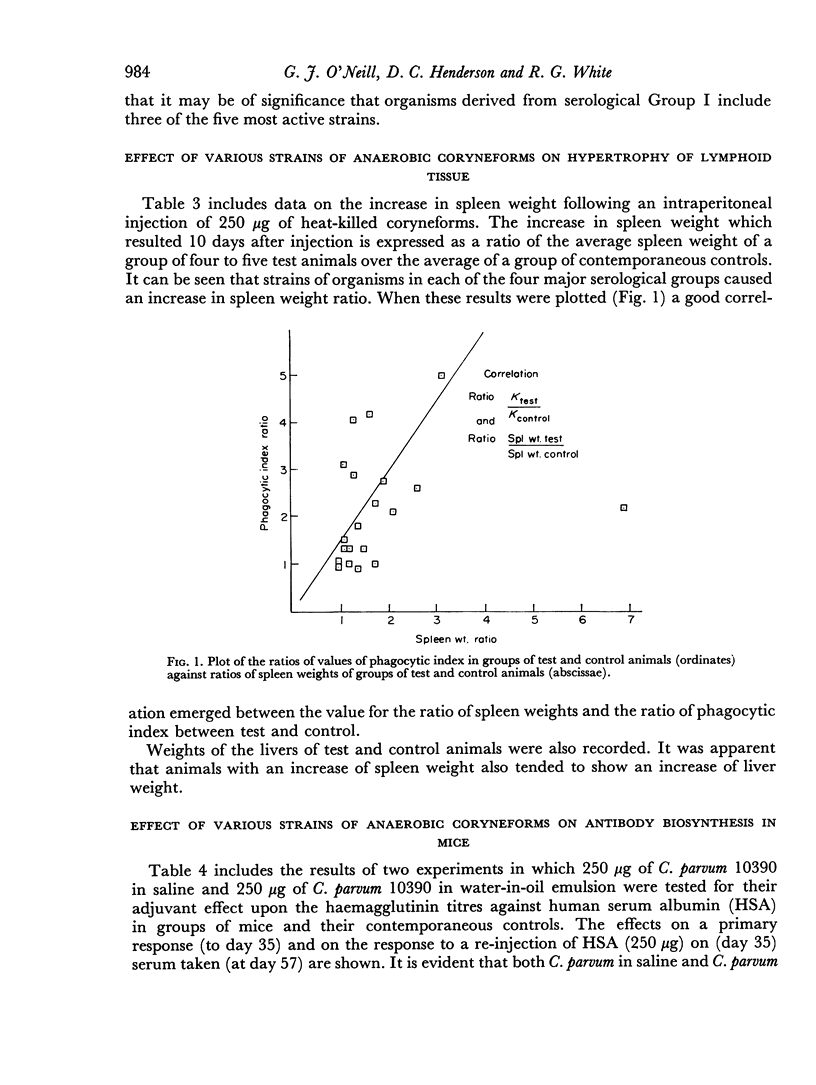
Many of the strains that stimulated a rise in phagocytic index also caused an increase in the weight of the spleen measured at 10 days after injection and a moderately good correlation was apparent between the magnitude of the two effects.
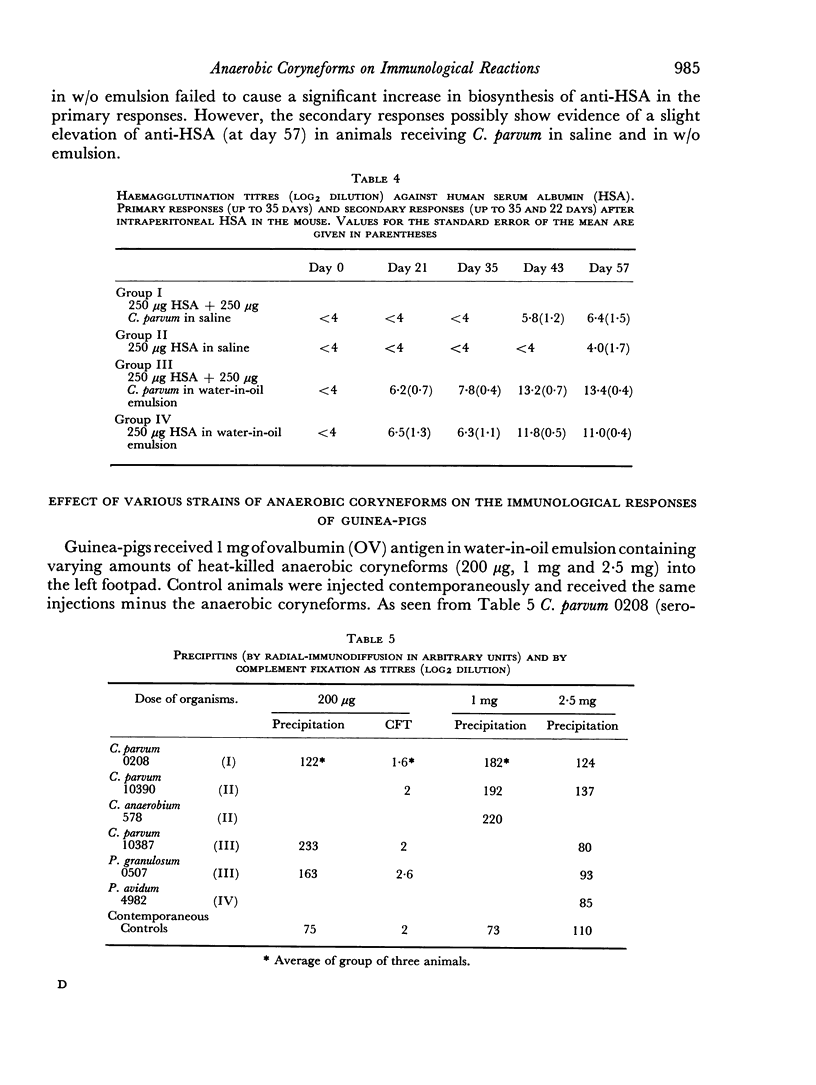
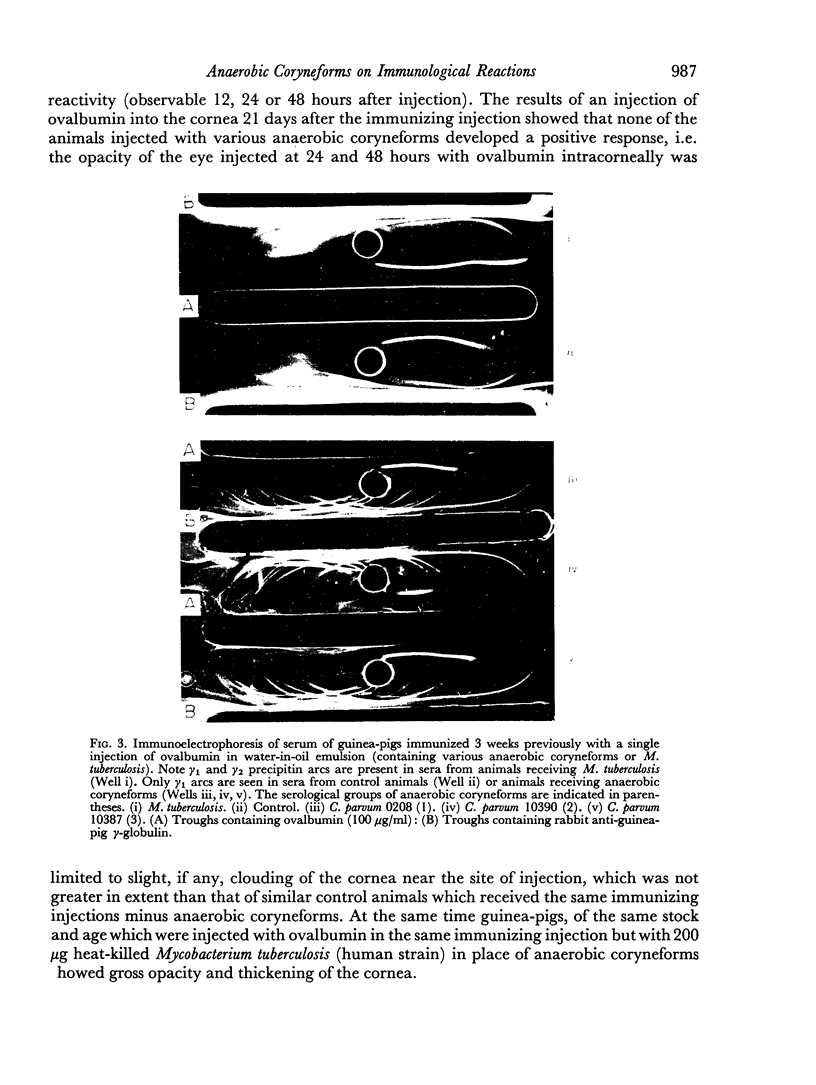
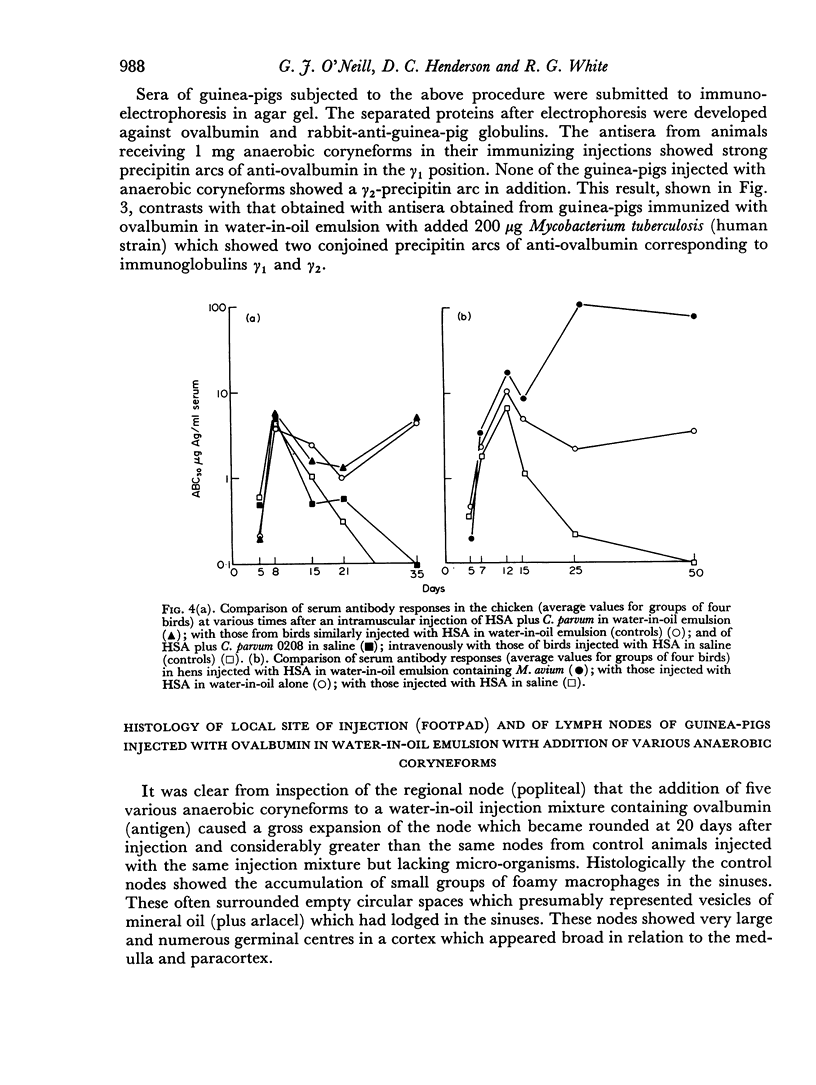
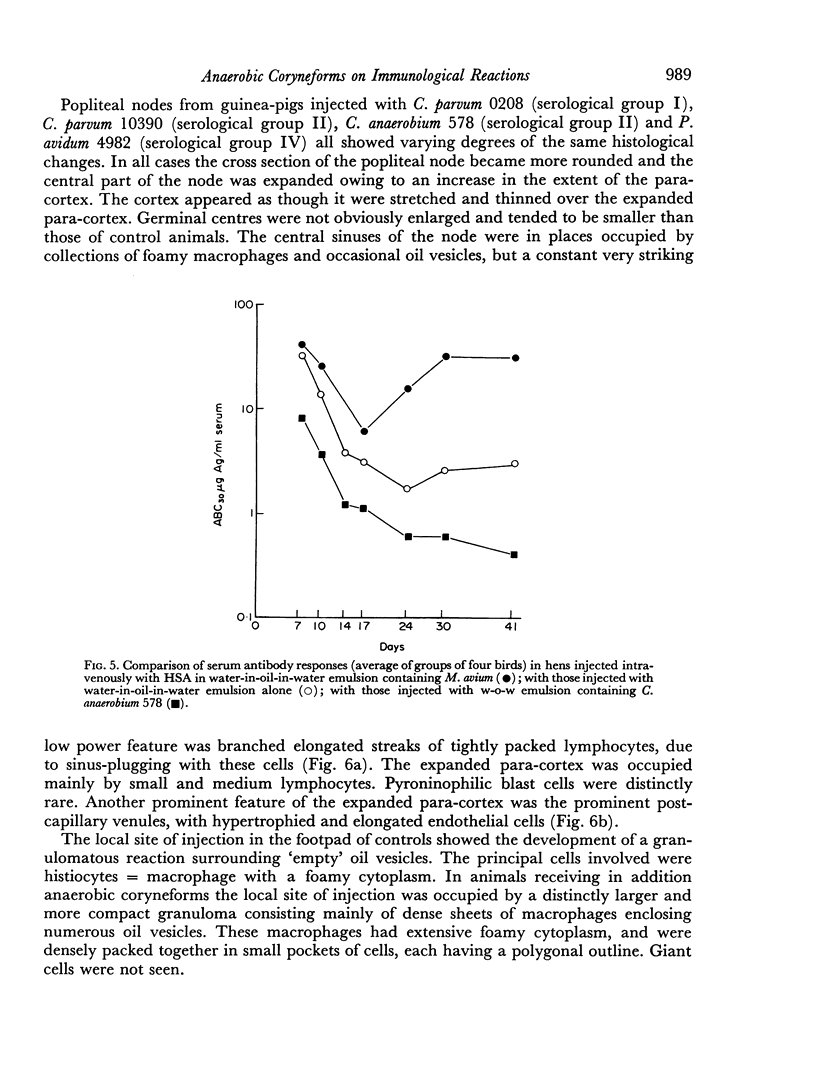
Tests in mice and chickens for an adjuvant action on serum levels of antibody failed to show any enhancement of primary responses, although a barely significant elevation of the secondary response in the mouse was observed. In the guinea-pig, a clear adjuvant effect on the levels of serum antibody in a primary response was observed. However, no evidence could be obtained that any of several anaerobic coryneforms was able to enhance cell-mediated hypersensitivity, as shown by delayed-type skin tests or corneal reactions.
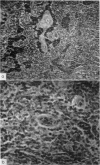
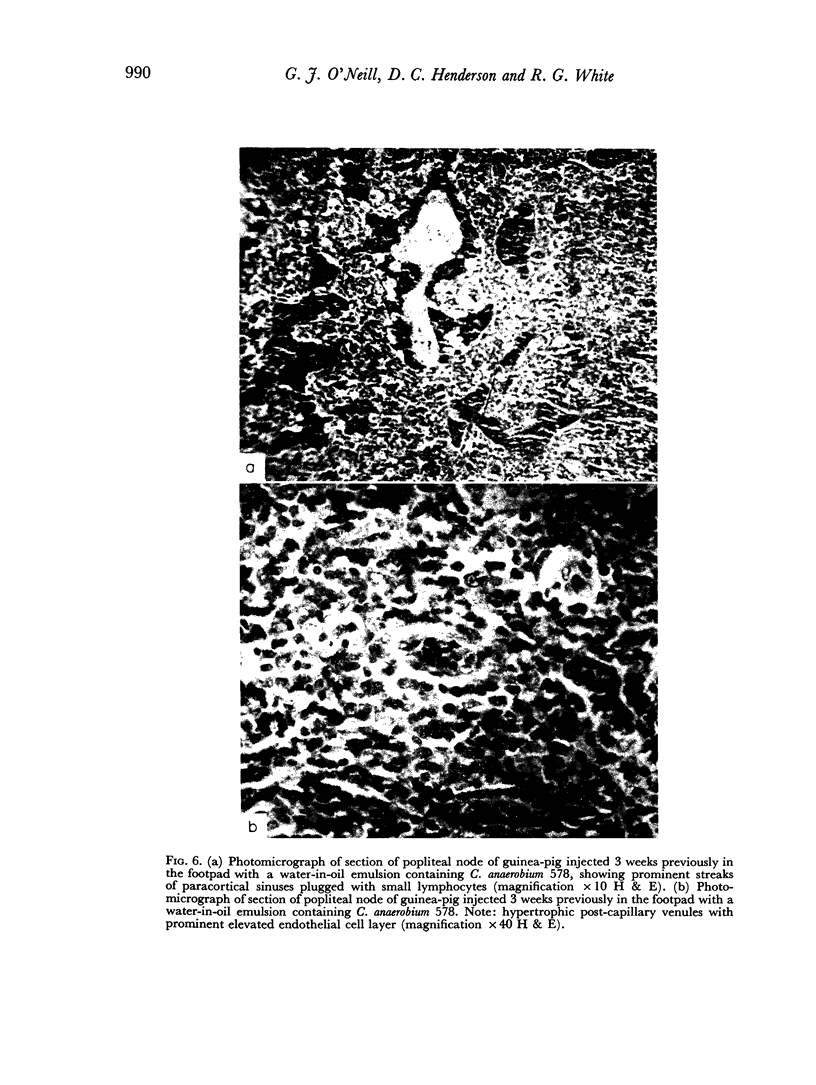
A study of the histological responses to a footpad injection of various anaerobic coryneforms (in water-oil emulsion with ovalbumin) showed that the regional (popliteal) node underwent a considerable expansion of the lymphoid-cell content of the paracortical (thymus-dependent) area. This was accompanied by extensive sinus plugging by lymphocytes and endothelial hypertrophy of post-capillary venules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Fisher J. C., Grace W. R., Mannick J. A. The effect of nonspecific immune stimulation with corynebacterium parvum on patterns of tumor growth. Cancer. 1970 Dec;26(6):1379–1382. doi: 10.1002/1097-0142(197012)26:6<1379::aid-cncr2820260630>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- French V. I., Stark J. M., White R. G. The influence of adjuvants on the immunological response of the chicken. II. Effects of Freund's complete adjuvant on later antibody production after a single injection of immunogen. Immunology. 1970 May;18(5):645–655. [PMC free article] [PubMed] [Google Scholar]
- HALPERN B. N., PREVOT A. R., BIOZZI G., STIFFEL C., MOUTON D., MORARD J. C., BOUTHILLIER Y., DECREUSEFOND C. STIMULATION DE L'ACTIVIT'E PHAGOCYTAIRE DU SYST'EME R'ETICULOENDOTH'ELIAL PROVOQU'EE PAR CORYNEBACTERIUM PARVUM. J Reticuloendothel Soc. 1964 Jan;1:77–96. [PubMed] [Google Scholar]
- Halpern B. N., Biozzi G., Stiffel C., Mouton D. Inhibition of tumour growth by administration of killed corynebacterium parvum. Nature. 1966 Nov 19;212(5064):853–854. doi: 10.1038/212853a0. [DOI] [PubMed] [Google Scholar]
- Halpern B., Fray A. Déclenchement de l'anémie hémolytique autoimmune chez de jeunes souriceaux NZB par l'administration de Corynebacterium parvum. Ann Inst Pasteur (Paris) 1969 Dec;117(6):778–789. [PubMed] [Google Scholar]
- Herbert W. J. Multiple emulsions. A new form of mineral-oil antigen adjuvant. Lancet. 1965 Oct 16;2(7416):771–771. doi: 10.1016/s0140-6736(65)90816-0. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Cummins C. S. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972 Mar;109(3):1047–1066. doi: 10.1128/jb.109.3.1047-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. H. Localization of afferent lymph cells within the draining node during a primary immune response. Nature. 1970 Aug 1;227(5257):510–513. doi: 10.1038/227510a0. [DOI] [PubMed] [Google Scholar]
- Kelly R. H., Wolstencroft R. A., Dumonde D. C., Balfour B. M. Role of lymphocyte activation products (LAP) in cell-mediated immunity. II. Effects of lymphocyte activation products on lymph node architecture and evidence for peripheral release of LAP following antigenic stimulation. Clin Exp Immunol. 1972 Jan;10(1):49–65. [PMC free article] [PubMed] [Google Scholar]
- McCracken A., McBride W. H., Weir D. M. Adjuvant-induced antired blood cell activity in CBA mice. Clin Exp Immunol. 1971 Jun;8(6):949–955. [PMC free article] [PubMed] [Google Scholar]
- NEVEU T., BRANELLEC A., BIOZZI G. PROPRI'ET'ES ADJUVANTES DE CORYNEBACTERIUM PARVUM SUR LA PRODUCTION D'ANTICORPS ET SUR L'INDUCTION DE L'HYPERSENSIBILIT'E RETARD'EE ENVERS LES PROT'EINES CONJUGU'EES. Ann Inst Pasteur (Paris) 1964 May;106:771–777. [PubMed] [Google Scholar]
- Smith L. H., Woodruff M. F. Comparative effect of two strains of C. parvum on phagocytic activity and tumour growth. Nature. 1968 Jul 13;219(5150):197–198. doi: 10.1038/219197a0. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., JENKINS G. C., WILKINSON P. C. The production of skin-sensitizing antibody in the guinea-pig. Int Arch Allergy Appl Immunol. 1963;22:156–165. doi: 10.1159/000229362. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F., Boak J. L. Inhibitory effect of injection of Corynebacterium parvum on the growth of tumour transplants in isogenic hosts. Br J Cancer. 1966 Jun;20(2):345–355. doi: 10.1038/bjc.1966.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa M. A., Parrott D. M. Induction and recall in contact sensivitity. Changes in skin and draining lymph nodes of intact and thymectomized mice. J Exp Med. 1969 Oct 1;130(4):671–690. doi: 10.1084/jem.130.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]