Abstract
The primary and secondary antibody response in inbred CBA mice against Escherichia coli O6 lipopolysaccharide was investigated. The avidity was found to be inversely related to the immunization dose. An optimally immunogenic dose resulted in a maximal IgM production on day 4 and a significant IgG production around day 30. Concomitant to the IgG formation there was an increase in avidity both at the serum level and at the level of the IgM-secreting cells.
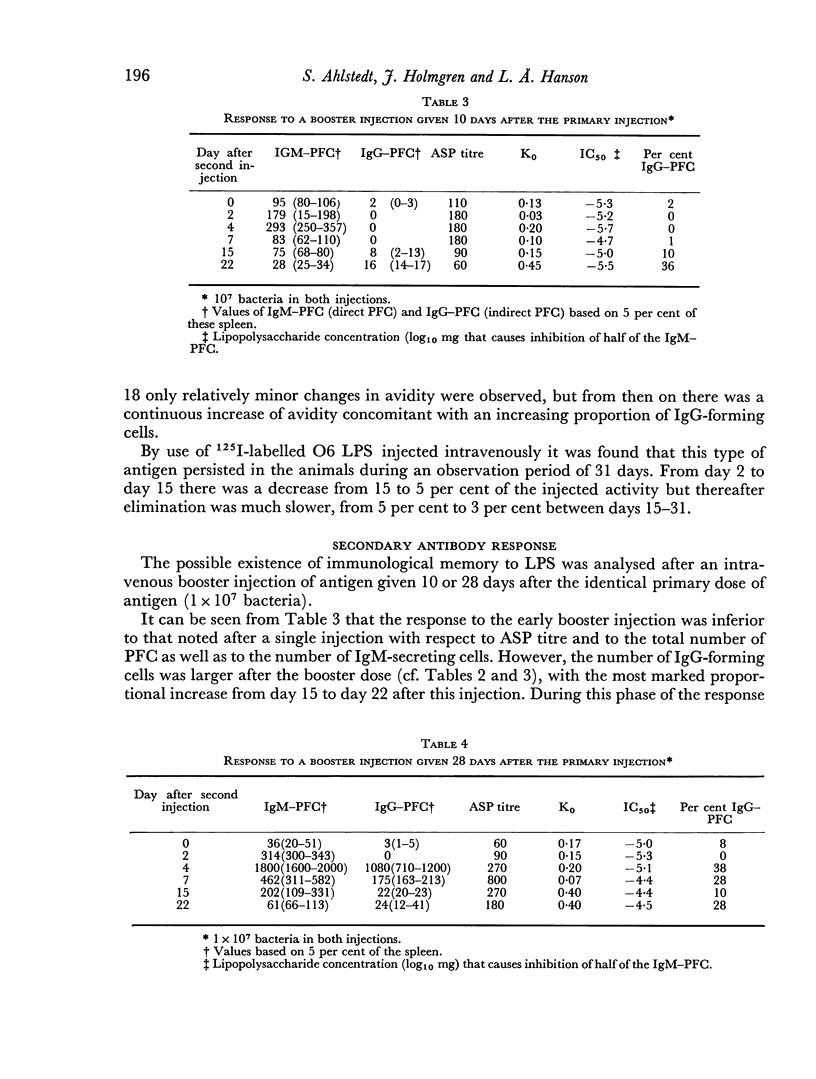
A time-dependent immunological memory was noted; a booster dose 28 days after priming gave rise to increased IgM and IgG antibody production as compared to the primary response while a booster dose 10 days after priming did not. The antibody avidity in the secondary response was similar to that in the primary response.
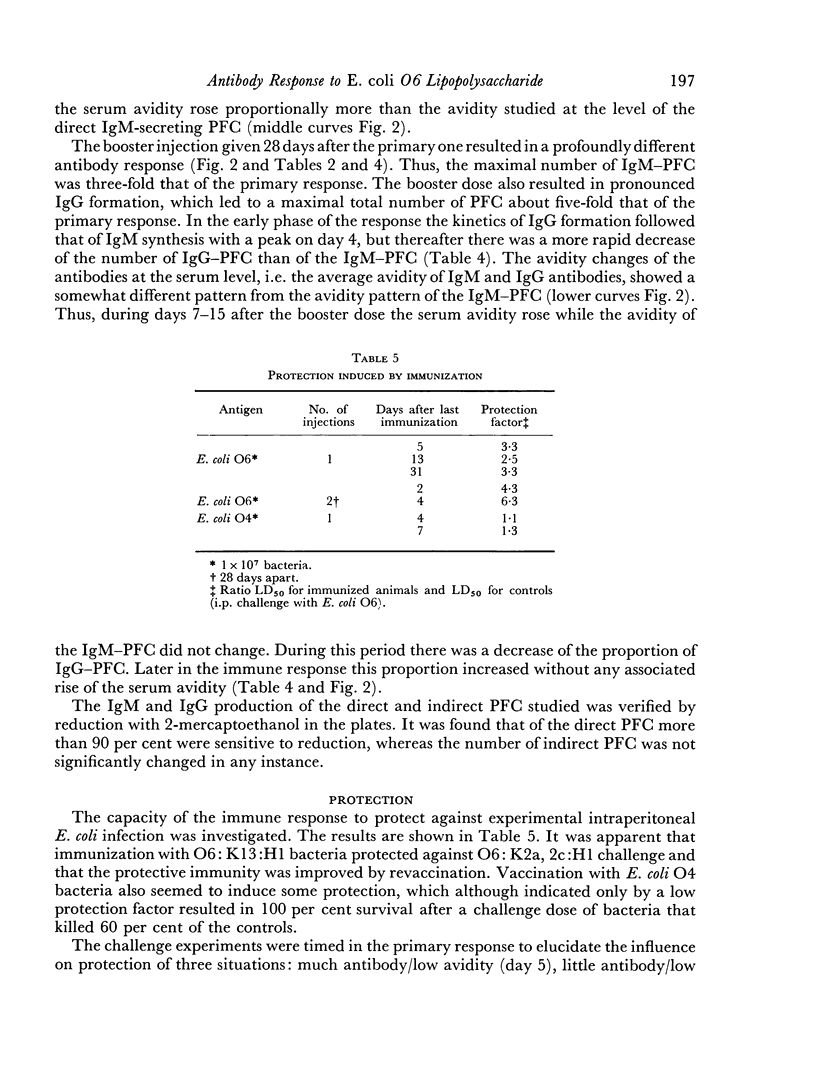
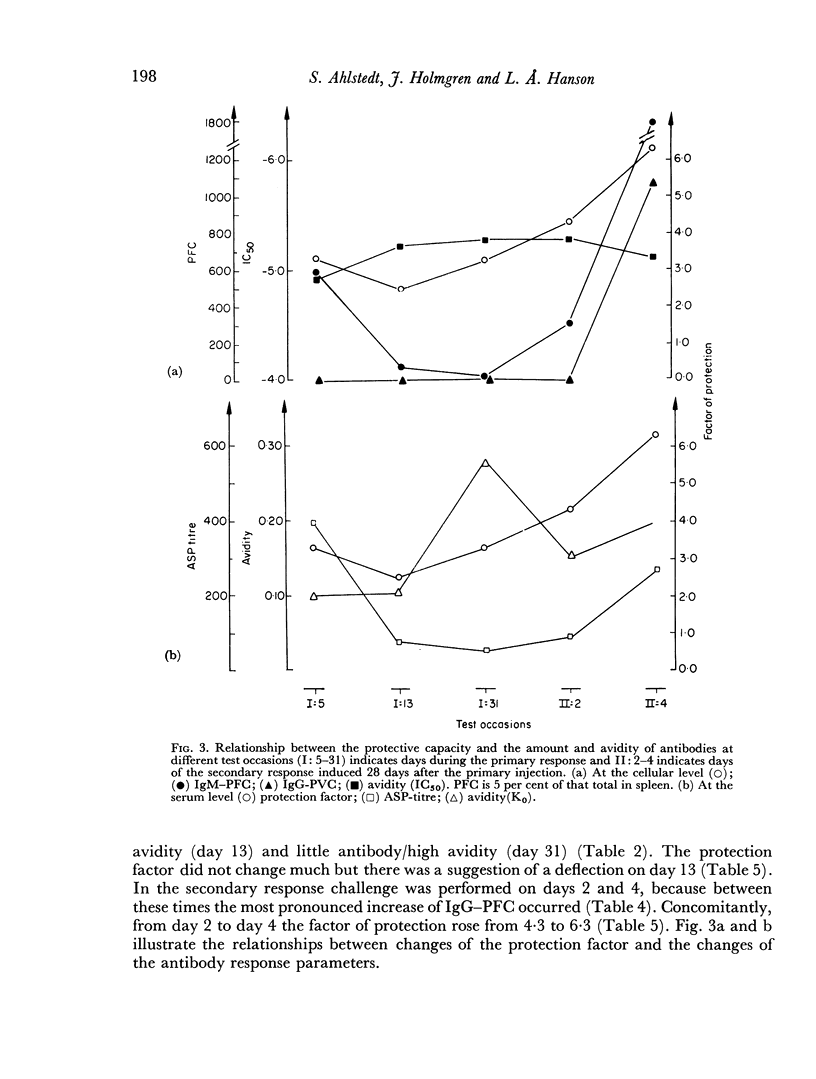
The protective capacity of the immune response seemed to be primarily related to the antibody level. However, high avidity of the IgG antibody population seemed to compensate partially for low amount.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Holmgren J., Hanson L. A. Significance of amount and avidity of E. coli O antibodies for manifestation of their serological and protective properties. A preliminary study. Int Arch Allergy Appl Immunol. 1972;42(6):826–835. doi: 10.1159/000230661. [DOI] [PubMed] [Google Scholar]
- Allen J. L., Friedman H. Induction of "low dose" tolerance to a bacterial somatic antigen in neonatal mice. Nat New Biol. 1971 Sep 15;233(37):82–84. doi: 10.1038/newbio233082a0. [DOI] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Prescott B., Stashak P. W., Amsbaugh D. F. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. 3. Studies on the average avidity of the antibody produced by specific plaque-forming cells. J Immunol. 1971 Sep;107(3):719–724. [PubMed] [Google Scholar]
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Cunningham A. J., Sercarz E. E. The asynchronous development of immunological memory in helper (T) and precursor (B) cell lines. Eur J Immunol. 1971 Dec;1(6):413–421. doi: 10.1002/eji.1830010602. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Holmgren J., Jodal U., Kaijser B. Characterization of precipitating antibodies to E. coli O antigen in infants and children with acute pyelonephritis. Clin Exp Immunol. 1971 Apr;8(4):573–580. [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Hanson L. A. Immunodiffusion studies on Escherichia coli. 2. Characterization of antigens used for passive haemagglutination with an O 6 strain as model. Acta Pathol Microbiol Scand. 1969;77(4):727–738. [PubMed] [Google Scholar]
- Holmgren J. The antibody response in rabbits to E. coli O antigen. Int Arch Allergy Appl Immunol. 1970;37(5):546–559. doi: 10.1159/000230242. [DOI] [PubMed] [Google Scholar]
- International Whaling Omission. Nature. 1971 Jul 9;232(5306):80–81. doi: 10.1038/232080a0. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., AVEGNO P. The development of the phage-inactivating properties of serum during the course of specific immunization of an animal: reversible and irreversible inactivation. J Immunol. 1956 Mar;76(3):200–208. [PubMed] [Google Scholar]
- Kaijser B., Holmgren J., Hanson L. A. The protective effect against E. coli of O and K antibodies of different immunoglobulin classes. Scand J Immunol. 1972;1(1):27–32. doi: 10.1111/j.1365-3083.1972.tb03732.x. [DOI] [PubMed] [Google Scholar]
- MULHOLLAND J. H., WOLFF S. M., JACKSON A. L., LANDY M. QUANTITATIVE STUDIES OF FEBRILE TOLERANCE AND LEVELS OF SPECIFIC ANTIBODY EVOKED BY BACTERIAL ENDOTOXIN. J Clin Invest. 1965 Jun;44:920–928. doi: 10.1172/JCI105209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Chemistry and reaction mechanisms of complement. Adv Immunol. 1968;8:1–80. doi: 10.1016/s0065-2776(08)60464-2. [DOI] [PubMed] [Google Scholar]
- Pasanen V. J. Effect of antigen dose on stimulation of anti-hapten plaque forming cells of different affinities. Int Arch Allergy Appl Immunol. 1971;40(1):153–160. doi: 10.1159/000230402. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas H., Mäkelä O. Haptenated bacteriophage in the assay of antibody quantity and affinity: maturation of an immune response. Immunochemistry. 1970 Nov;7(11):933–943. doi: 10.1016/0019-2791(70)90054-6. [DOI] [PubMed] [Google Scholar]
- Sjöberg O. Antigen-binding cells in mice immune or tolerant to Escherichia coli polysaccharide. J Exp Med. 1971 May 1;133(5):1015–1025. doi: 10.1084/jem.133.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Eisen H. N. The relative affinity of antibodies synthesized in the secondary response. J Exp Med. 1967 Dec 1;126(6):1185–1205. doi: 10.1084/jem.126.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis G. A., Siskind G. W. Selection of cell populations in induction of tolerance: affinity of antibody formed in partially tolerant rabbits. J Immunol. 1968 Jan;100(1):138–141. [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]


