Abstract
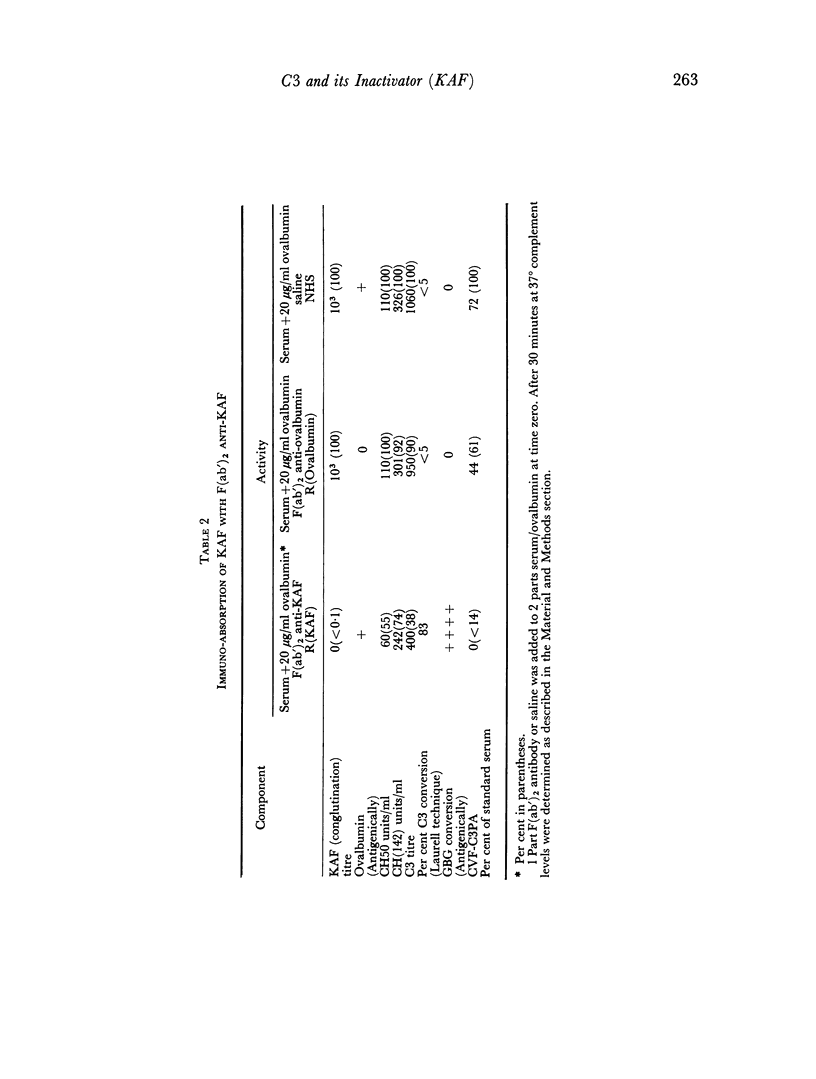
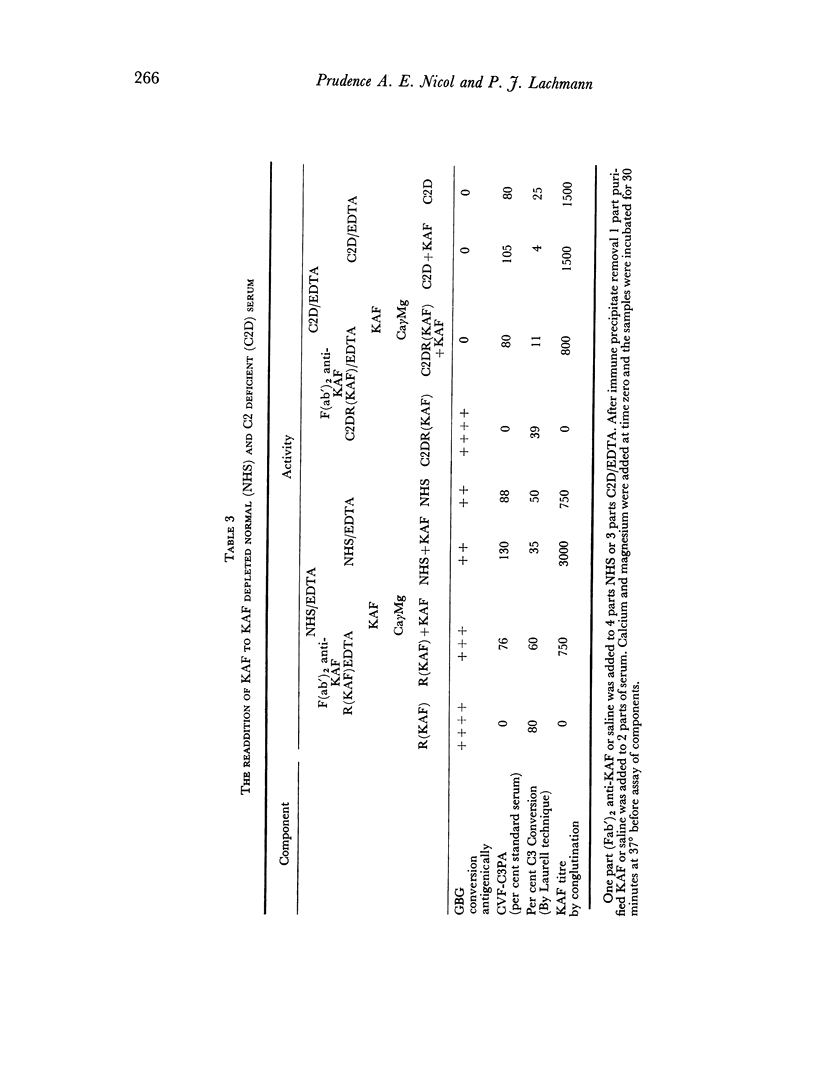
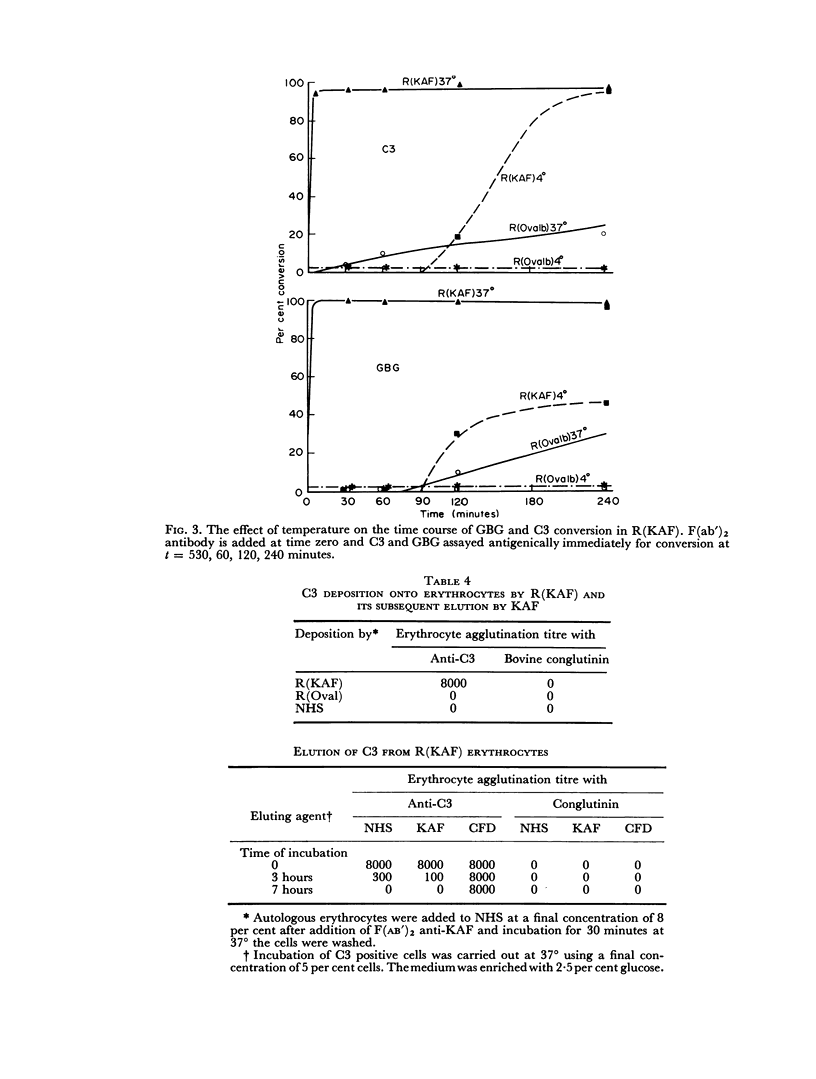
The immunochemical depletion from serum of C3b inactivator (KAF) has been performed using purified F(ab′)2 antibody. In vitro KAF depletion leads to spontaneous activation of the `alternate pathway of complement fixation' as evidenced by conversion of glycine-rich β-glycoprotein, depletion of cobra venom factor—C3 proactivator and conversion of C3.
The status of the complement system following in vitro KAF depletion accurately mimics that found in vivo in the unique KAF-deficient patient.
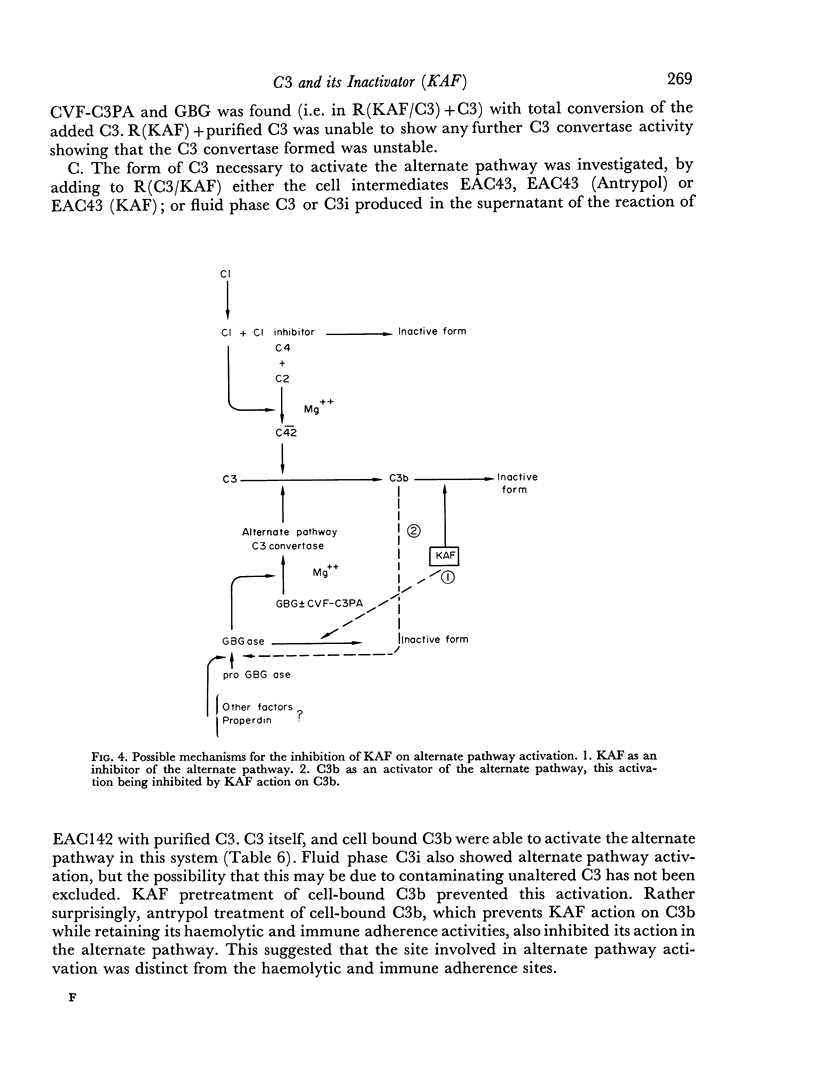
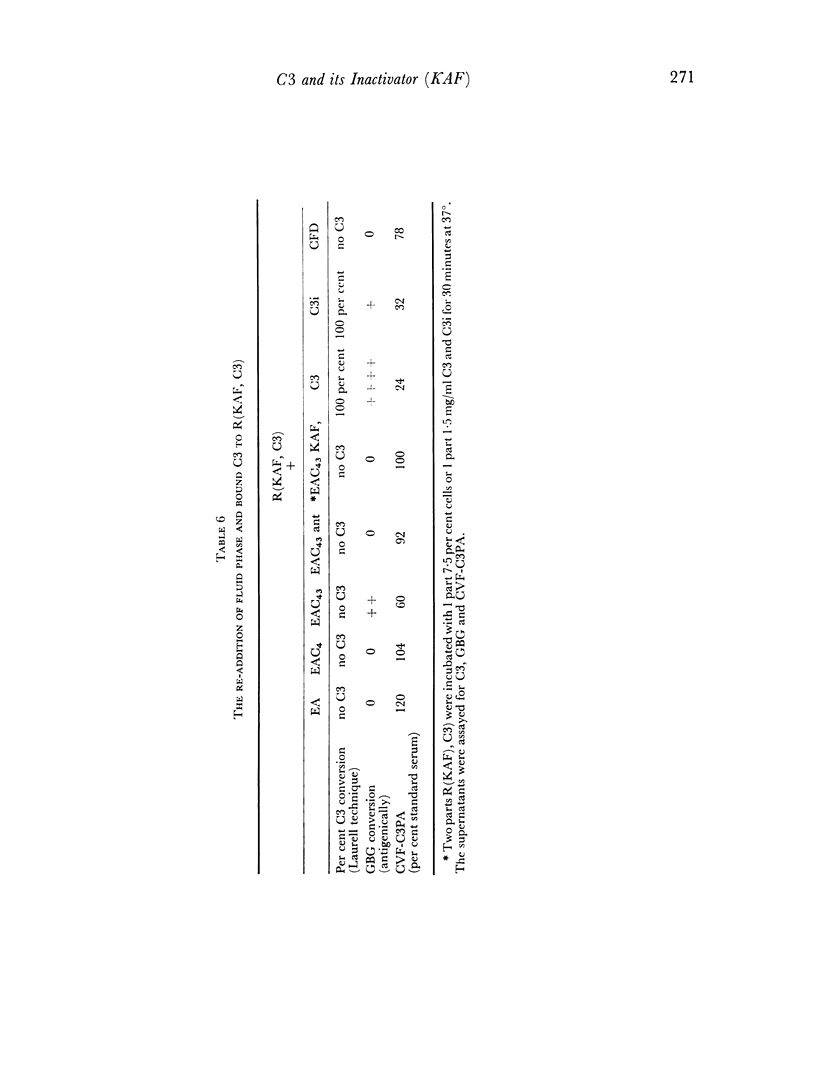
The activation of the alternative pathway whether by KAF depletion or by conventional alternative pathway activators is prevented by the immunochemical depletion of C3. It therefore appears that the alternative pathway is essentially a C3b-feedback pathway which is normally controlled by the activity of KAF.
Full text
PDF



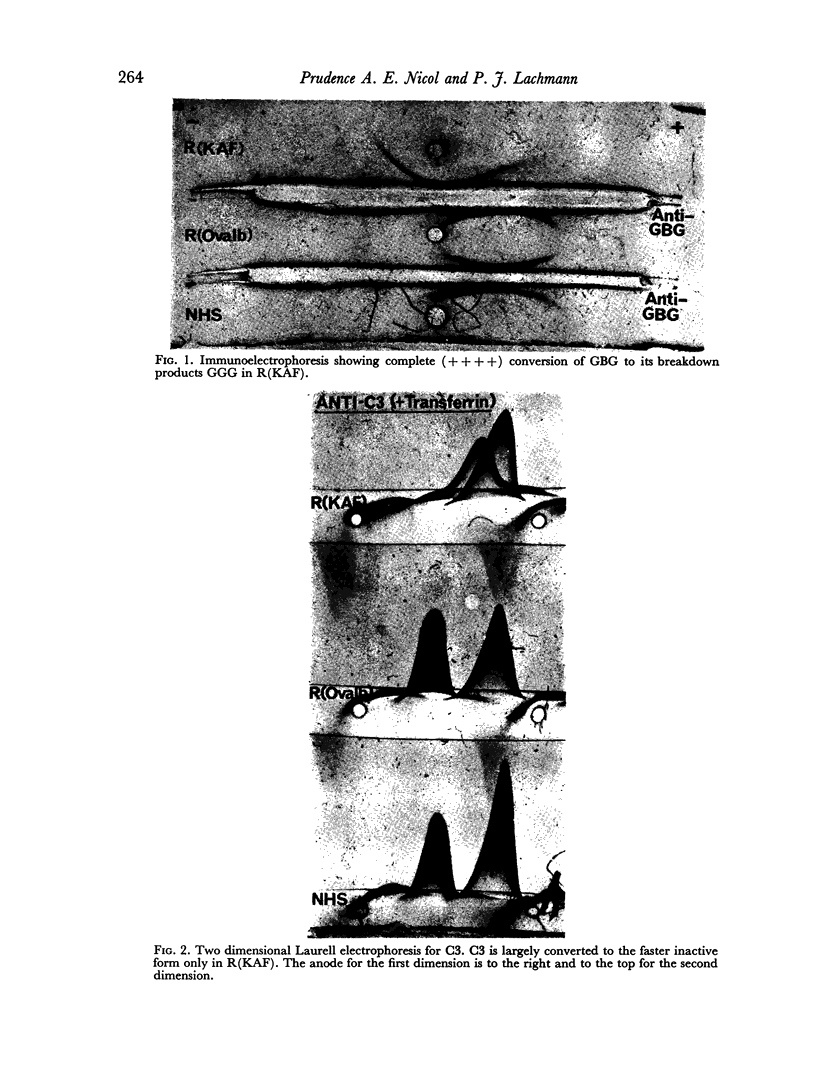







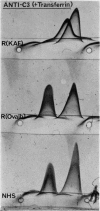
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Alper C. A., Lachmann P. J., Rosen F. S., Jandl J. H. Deficiency of C3 inactivator in man. J Immunol. 1971 Jul;107(1):19–27. [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Boenisch T., Watson L. Genetic polymorphism in human glycine-rich beta-glycoprotein. J Exp Med. 1972 Jan;135(1):68–80. doi: 10.1084/jem.135.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S., Lachmann P. J. Inactivator of the third component of complement as an inhibitor in the properdin pathway. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2910–2913. doi: 10.1073/pnas.69.10.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUM L., PILLEMER L., LEPOW I. H. The properdin system and immunity. XIII. Assay and properties of a heat-labile serum factor (factor B) in the properdin system. Z Immun exp ther. 1959 Oct-Nov;118:349–357. [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Boenisch T., Alper C. A. Isolation and properties of a glycine-rich beta-glycoprotein of human serum. Biochim Biophys Acta. 1970 Dec 22;221(3):529–535. doi: 10.1016/0005-2795(70)90224-2. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. C3 leukotactic factors produced by a tissue protease. J Exp Med. 1969 Sep 1;130(3):505–518. doi: 10.1084/jem.130.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Liske R. The preparation and properties of alexinated intermediates that react with conglutinin. I. Guinea-pig complement. Immunology. 1966 Sep;11(3):243–254. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Lachmann P. J. The purification of specific antibody as F(ab')2 by the pepsin digestion of antigen-antibody precipitates, and its application to immunoglobulin and complement antigens. Immunochemistry. 1971 Jan;8(1):81–88. doi: 10.1016/0019-2791(71)90423-x. [DOI] [PubMed] [Google Scholar]
- Marcus R. L., Shin H. S., Mayer M. M. An alternate complement pathway: C-3 cleaving activity, not due to C4,2a, on endotoxic lipopolysaccharide after treatment with guinea pig serum; relation to properdin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1351–1354. doi: 10.1073/pnas.68.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Fjellström K. E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J Immunol. 1971 Dec;107(6):1666–1672. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr A new concept of immunosuppression in hypersensitivity reactions and in transplantation immunity. Surv Ophthalmol. 1966 Aug;11(4):498–505. [PubMed] [Google Scholar]
- PENSKY J., WURZ L., PILLEMER L., LEPOW I. H. The properdin system and immunity. XII. Assay, properties and partial purification of a hydrazine-sensitive serum factor (factor A) in the properdin system. Z Immun exp ther. 1959 Oct-Nov;118:329–348. [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Complement fixation by the F(ab')2-fragment of pepsin-treated rabbit antibody. Immunology. 1971 May;20(5):649–658. [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Ruddy S., Hunsicker L. G., Austen K. F. C3b inactivator of man. 3. Further purification and production of antibody to C3b INA. J Immunol. 1972 Mar;108(3):657–664. [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]
- Thompson R. A. C3 inactivating factor in the serum of a patient with chronic hypocomplementaemic proliferative glomerulo-nephritis. Immunology. 1972 Jan;22(1):147–158. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. A plasmin-split fragment of C'3 as a new chemotactic factor. J Exp Med. 1967 Aug 1;126(2):189–206. doi: 10.1084/jem.126.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C., Davis N. C., Forristal J., Herbst J., Spitzer R. Antigenic determinants of human beta-1c and beta-1g-globulins. J Immunol. 1966 Apr;96(4):650–658. [PubMed] [Google Scholar]