Abstract
[125I]iododeoxyuridine was used to assess the DNA synthesis in the lymph nodes and spleen of mice exposed to picrylating agents. Skin painting with picryl chloride causes both contact (delayed) hypersensitivity and antibody production. It also increases DNA synthesis in the draining lymph nodes and spleen. The lymph node response peaks at day 3 and reverts to normal by day 7. In contrast, the DNA synthesis in the spleen shows three well-defined peaks on days 2, 5 and 8. The total DNA synthesis in the first 4 days is 0.6–2.4 times greater in the spleen than in the draining lymph nodes.
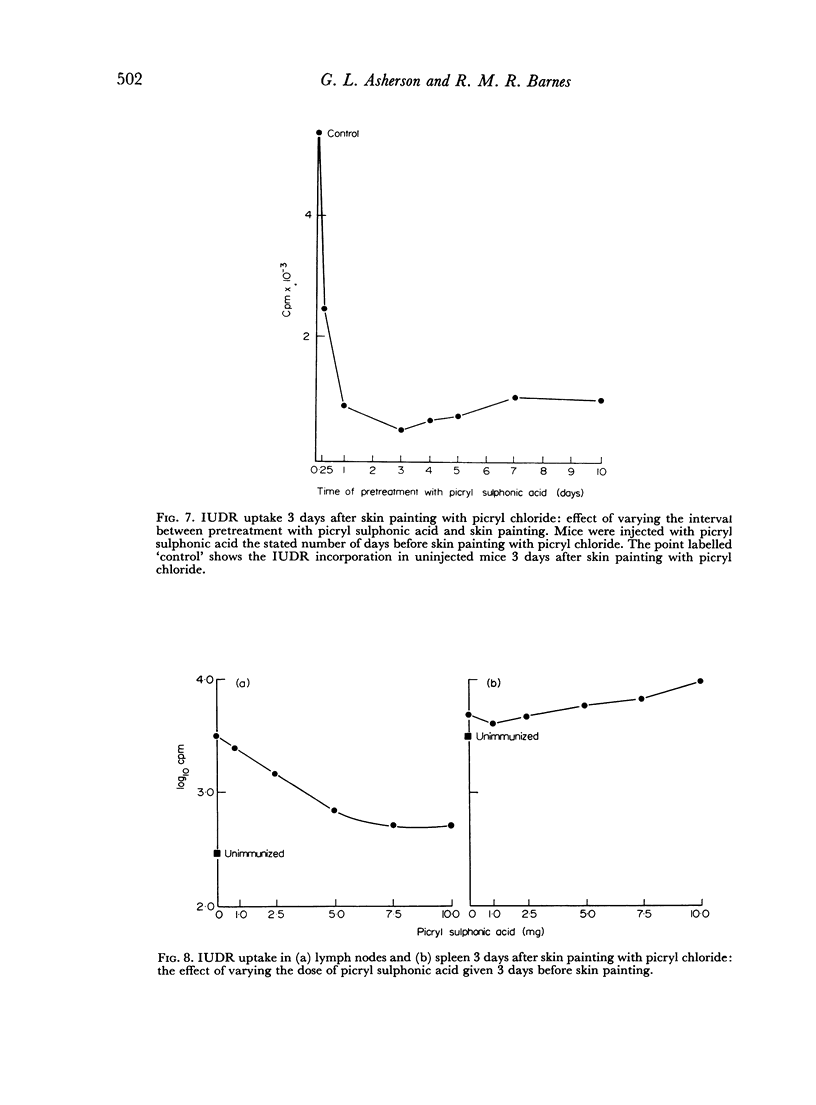
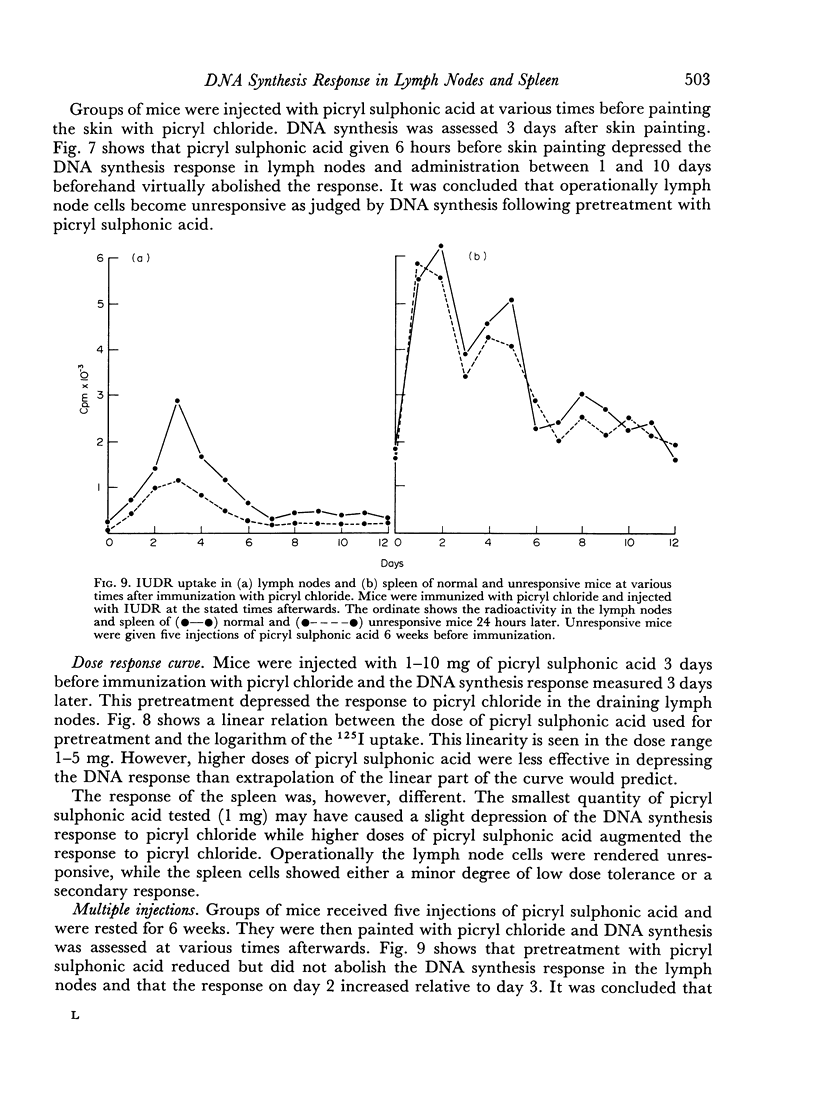
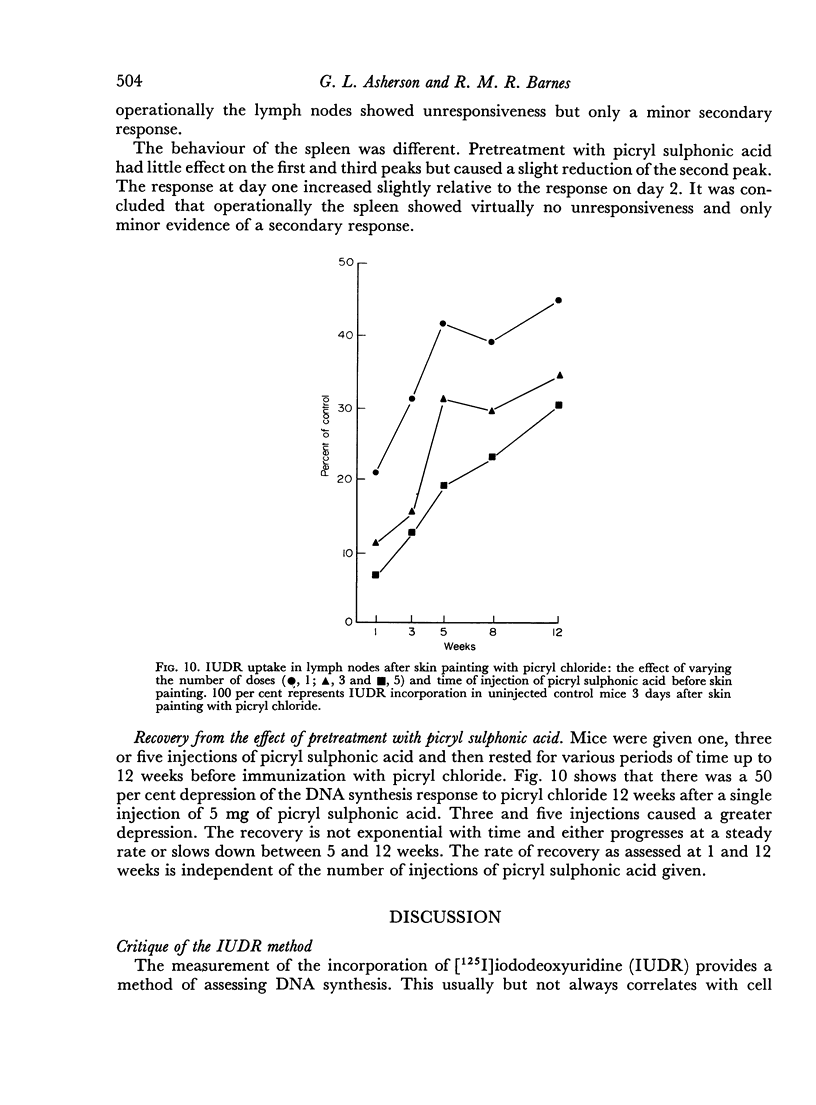
It is known that the intravenous injection of picryl sulphonic acid abolishes the contact sensitivity which otherwise follows skin painting with picryl chloride but has less effect on antibody production. This pretreatment depresses and may virtually abolish the DNA synthesis response of the lymph nodes to skin painting with picryl chloride. In contrast the response in the spleen is almost unaltered in magnitude but the first peak occurs somewhat earlier. This indicates that cells in one anatomical location, the lymph nodes, may behave as though they were tolerant while cells in another location, the spleen, behave as though they were immune.
The intravenous injection of picryl sulphonic acid causes DNA synthesis in the lymph nodes and spleen. The response in the lymph nodes but not in the spleen is reduced by pretreatment with this agent. This is an example of DNA synthesis following exposure to an antigen which causes unresponsiveness.
Full text
PDF

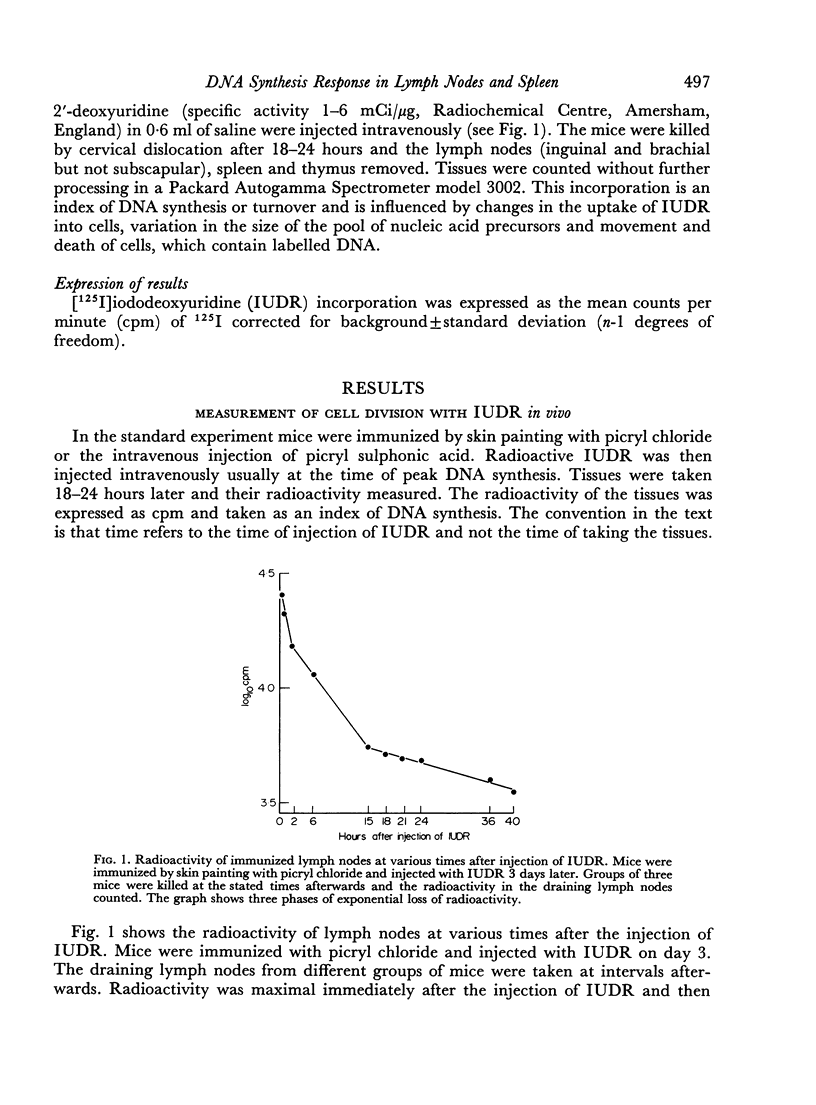
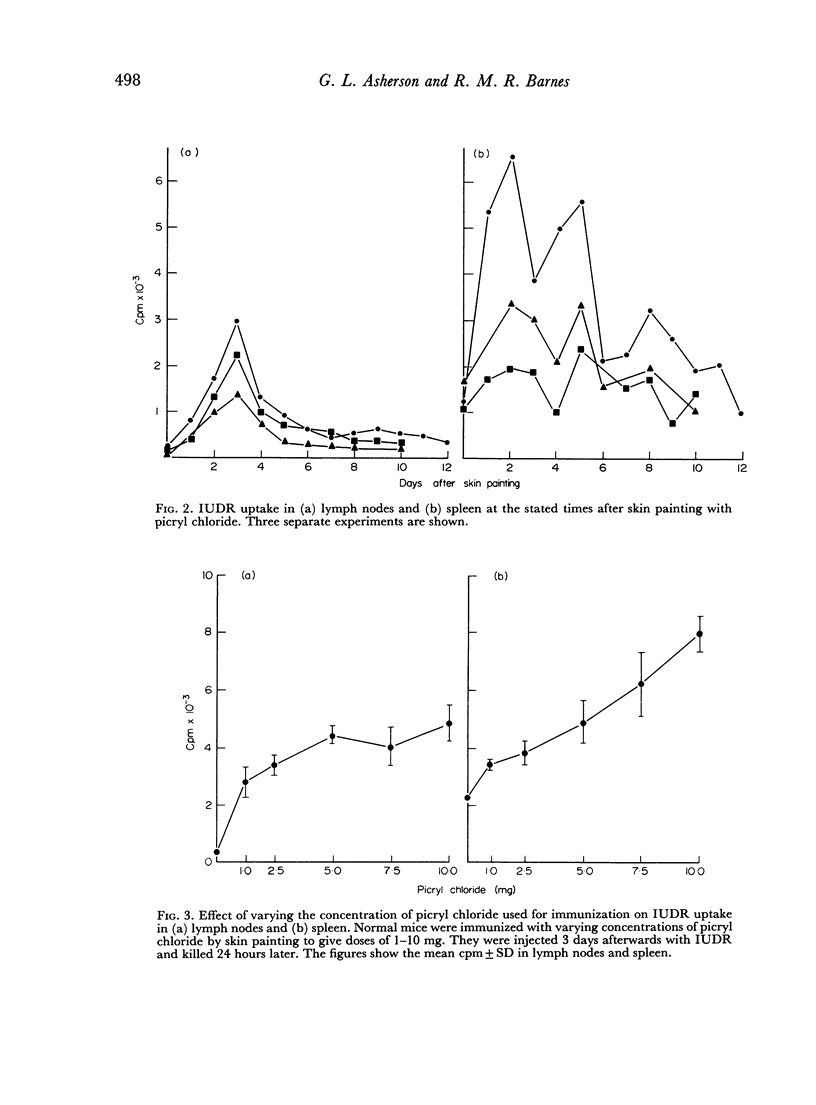

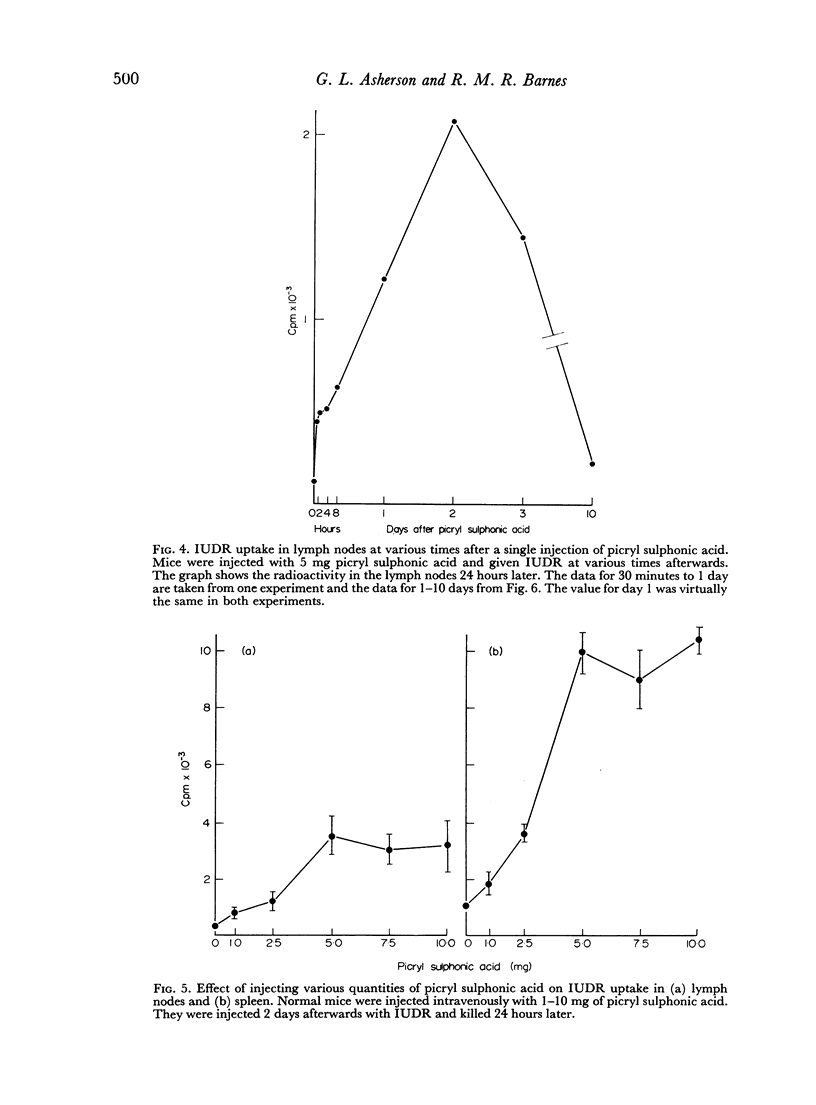
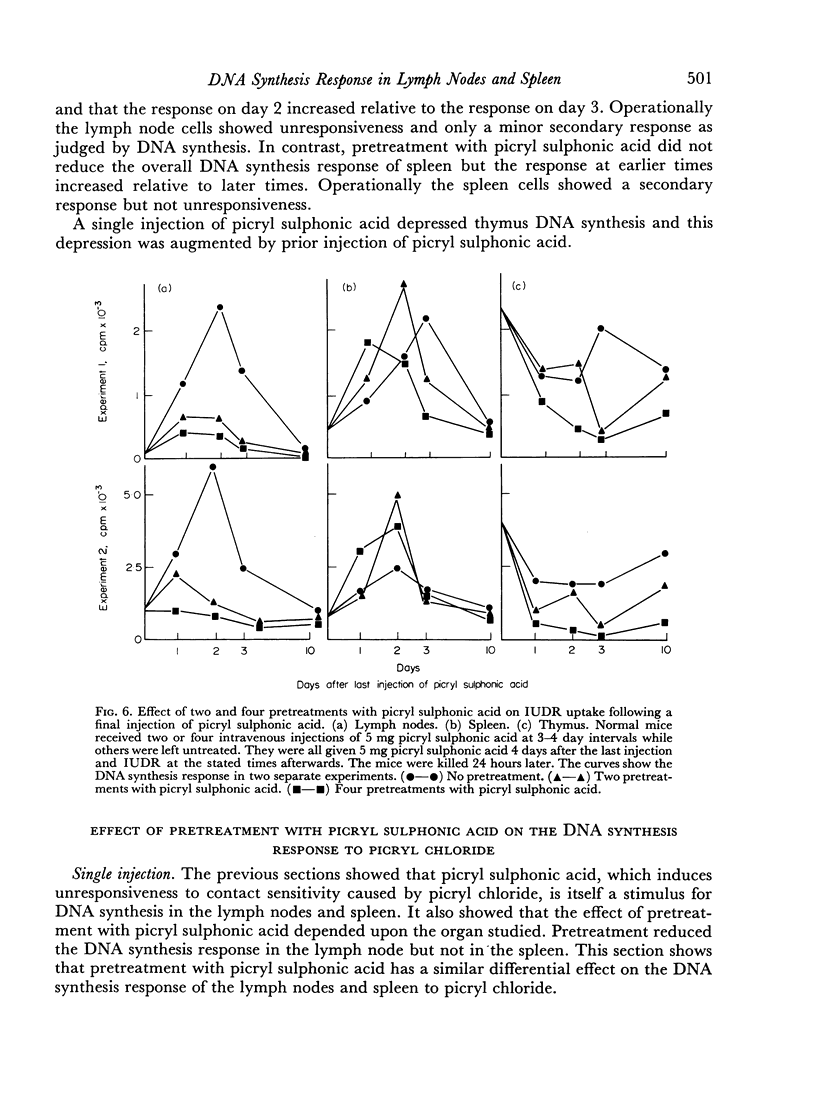




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L. Antigen-mediated depression of delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):24–29. doi: 10.1093/oxfordjournals.bmb.a070510. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Ferluga J. Contact sensitivity in the mouse. X. Non-specific cytotoxicity of T blasts in the draining lymph nodes. Immunology. 1973 Sep;25(3):471–483. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Ptak W. Contact and delayed hypersensitivity in the mouse. 3. Depression of contact sensitivity by pre-treatment with antigen and the restoration of immune competence in tolerant mice by normal lymphoid and bone marrow cells. Immunology. 1970 Jan;18(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Stone S. H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: description of the phenomenon and the effect of splenectomy. Immunology. 1965 Sep;9(3):205–217. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Barnes R. M. The mechanism of immunological unresponsiveness to picryl chloride and the possible role of antibody mediated depression. Clin Exp Immunol. 1971 Jul;9(1):111–121. [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Asherson G. L. Contact sensitivity to oxazolone in the mouse. VIII. Demonstration of several classes of antibody in the sera of contact sensitized and unimmunized mice by a simplified antiglobulin assay. Immunology. 1972 Sep;23(3):289–298. [PMC free article] [PubMed] [Google Scholar]
- Cantor H. T cells and the immune response. Prog Biophys Mol Biol. 1972;25:73–82. doi: 10.1016/0079-6107(72)90016-8. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Hu C. C. Adoptive transfer of immunologic tolerance into normal mice. J Immunol. 1969 Dec;103(6):1242–1247. [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V. The morphology of immune reactions in normal, thymectomized and reconstituted mice. II. The response to oxazolone. Immunology. 1969 Jul;17(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Flax M. H. Immunologic unresponsiveness in the adult guinea pig. II. The kinetics of unresponsiveness. J Immunol. 1966 Mar;96(3):546–553. [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- HUGHES W. L., COMMERFORD S. L., GITLIN D., KRUEGER R. C., SCHULTZE B., SHAH V., REILLY P. DEOXYRIBONUCLEIC ACID METABOLISM IN VIVO: I. CELL PROLIFERATION AND DEATH AS MEASURED BY INCORPORATION AND ELIMINATION OF IODODEOXYURIDINE. Fed Proc. 1964 May-Jun;23:640–648. [PubMed] [Google Scholar]
- HUMPHREY J. H. IMMUNOLOGICAL UNRESPONSIVENESS TO PROTEIN ANTIGENS IN RABBITS. I. THE DURATION OF UNRESPONSIVENESS FOLLOWING A SINGLE INJECTION AT BIRTH. Immunology. 1964 Jul;7:449–461. [PMC free article] [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Loewi G., Holborow E. J., Temple A. Inhibition of delayed hypersensitivity by pre-immunization without complete adjuvant. Immunology. 1966 Apr;10(4):339–347. [PMC free article] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Mitchison N. A. The effect of antigen dosage on the response of adoptively transferred cells. Immunology. 1965 Jun;8(6):549–556. [PMC free article] [PubMed] [Google Scholar]
- Parish C. R. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. J Exp Med. 1971 Jul 1;134(1):21–47. doi: 10.1084/jem.134.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc S. R., Harris G., Caldwell I. The relationship between antibody formation and deoxyribonucleic acid (DNA) synthesis in mouse spleen during primary and secondary response to sheep erythrocytes (SRC). Immunology. 1972 Aug;23(2):183–197. [PMC free article] [PubMed] [Google Scholar]
- Pollack S. B. Effect of host sex and splenectomy on moloney virus-induced sarcomas. Int J Cancer. 1971 Sep 15;8(2):264–271. doi: 10.1002/ijc.2910080211. [DOI] [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. B. AN EFFECT OF THYMECTOMY ON RECOVERY FROM IMMUNOLOGICAL PARALYSIS. Immunology. 1964 Sep;7:595–602. [PMC free article] [PubMed] [Google Scholar]
- Taub R. N., Gershon R. K. The effect of localized injection of adjuvant material on the draining lymph node. 3. Thymus dependence. J Immunol. 1972 Feb;108(2):377–386. [PubMed] [Google Scholar]


