Abstract
An earlier paper showed that 4 days after immunization with the contact sensitizing agent `oxazolone' there is a peak in the percentage of cells in the draining lymph nodes that move to sites of inflammation. This was assessed by dissociating the lymph nodes, labelling them with 51Cr and injecting them into mice whose ears were painted within an hour with an unrelated contact sensitizing agent or with croton oil. The ears were then removed at about 18 hours and their radioactivity used as a measure of cell arrival.
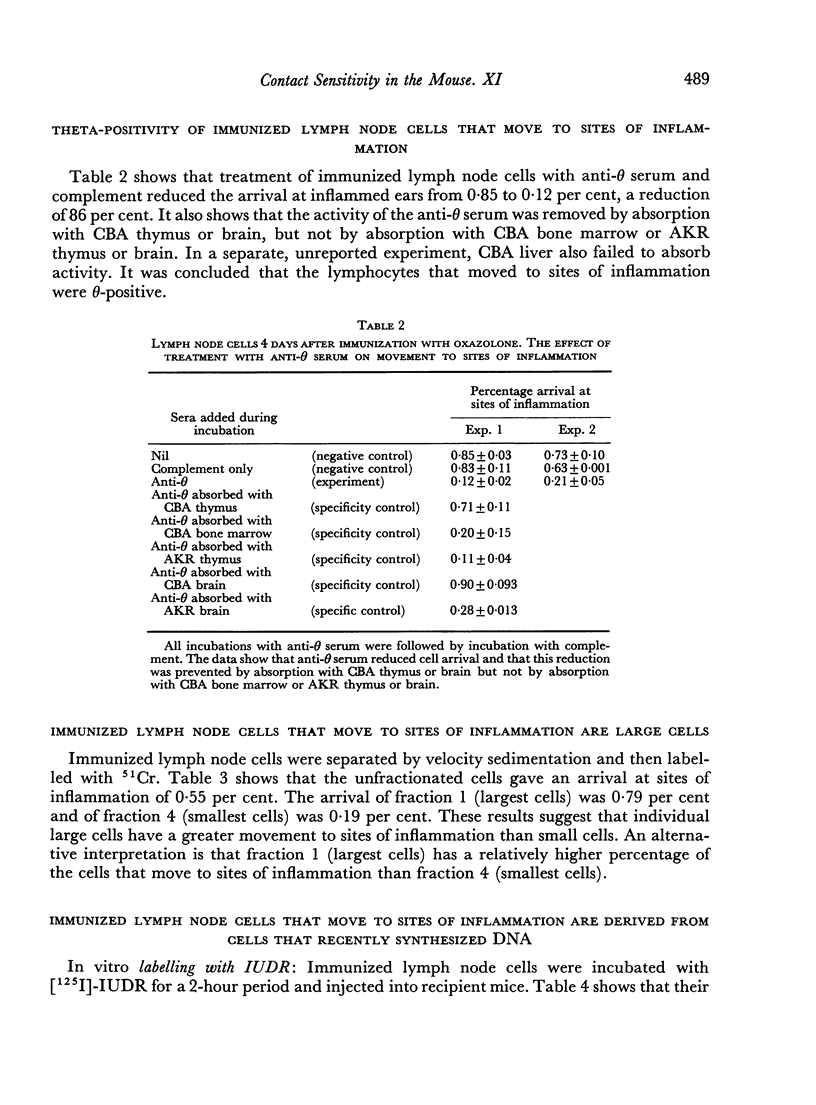
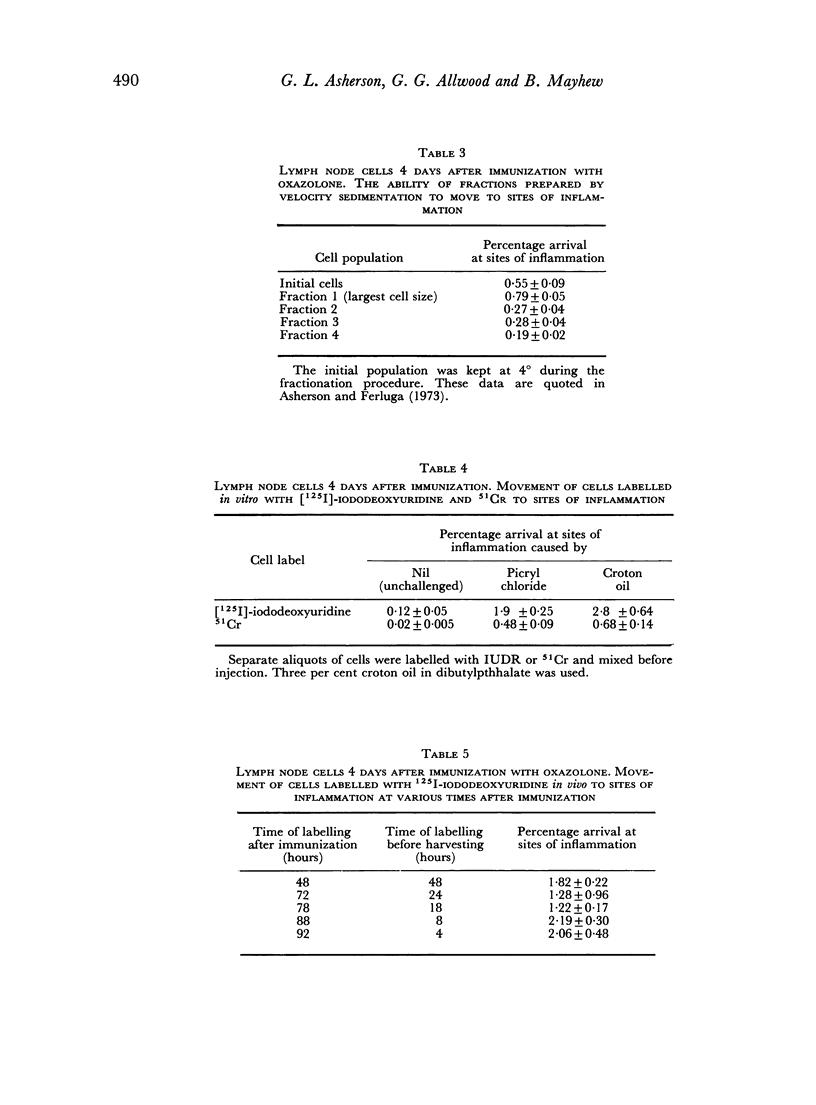
The cells that move to sites of inflammation are not macrophages as they are not removed by filtration through cotton wool. They are θ-positive and control studies show that the activity of the anti-θ serum was indeed due to antibody to the θ-antigen. The cells are large and can be labelled both in vivo and in vitro with [125I]-iododeoxyuridine which is incorporated the DNA of cells during the S phase of the cell mitotic cycle. It was concluded that the cells in immunized lymph nodes that move to sites of inflammation are T blasts. The unitary hypothesis is put forward that following immunization for delayed hypersensitivity a particular class of T cells proliferates and gives rise to large pyroninophilic blast cells; and that these cells or their immediate descendants possess the properties, either at the same time or at closely related times, of movement to sites of inflammation and non-specific cytotoxicity as well as the capacity to passively transfer cellular immunity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Bensted J., Delorme E. J., Hall J. G., Hodgett J. The cellular immune response to primary sarcomata in rats. II. Abnormal responses of nodes draining the tumour. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):237–251. doi: 10.1098/rspb.1969.0090. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Barnes R. M. Contact sensitivity in the mouse. XII. The use of DNA synthesis in vivo to determine the anatomical location of immunological unresponsiveness to picryl chloride. Immunology. 1973 Sep;25(3):495–508. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Ferluga J. Contact sensitivity in the mouse. X. Non-specific cytotoxicity of T blasts in the draining lymph nodes. Immunology. 1973 Sep;25(3):471–483. [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V. The morphology of immune reactions in normal, thymectomized and reconstituted mice. II. The response to oxazolone. Immunology. 1969 Jul;17(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Delorme E. J., Hodgett J., Hall J. G., Alexander P. The cellular immune response to primary sarcomata in rats. I. The significance of large basophilic cells in the thoracic duct lymph following antigenic challenge. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):229–236. doi: 10.1098/rspb.1969.0089. [DOI] [PubMed] [Google Scholar]
- Dennert G., Lennox E. Cell interactions in humoral and cell-mediated immunity. Nat New Biol. 1972 Jul 26;238(82):114–116. doi: 10.1038/newbio238114a0. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. THE ROLE OF LYMPHOCYTES IN THE DESTRUCTION OF HOMOGRAFTS. Br Med Bull. 1965 May;21:106–110. doi: 10.1093/oxfordjournals.bmb.a070376. [DOI] [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. I. Rapid method for isolation of theta-positive antigen-stimulated cells. Immunology. 1972 Jun;22(6):959–965. [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D. The mediator of cellular immunity. 3. Lymphocyte traffic from the blood into the inflamed peritoneal cavity. J Exp Med. 1971 Apr 1;133(4):864–876. doi: 10.1084/jem.133.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Asherson G. L. Contact and delayed hypersensitivity in the mouse. II. The role of different cell populations. Immunology. 1969 Nov;17(5):769–775. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Blake J. T., Rosenthal A. S. The peritoneal exudate lymphocyte. I. Differences in antigen responsiveness between peritoneal exudate and lymph node lymphocytes from immunized guinea pigs. J Exp Med. 1971 Nov 1;134(5):1170–1186. doi: 10.1084/jem.134.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. 3. Immunological characteristics of recirculating lymphocytes derived from activated thymus cells. Cell Immunol. 1972 Feb;3(2):213–230. doi: 10.1016/0008-8749(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. I. Quantitation of the proliferative response of thymus cells. Cell Immunol. 1972 Mar;3(3):361–384. doi: 10.1016/0008-8749(72)90244-4. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. II. Recirculating lymphocytes derived from antigen-activated thymus cells. Cell Immunol. 1972 Mar;3(3):385–404. doi: 10.1016/0008-8749(72)90245-6. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Cytology of the induction of hypersensitivity. Br Med Bull. 1967 Jan;23(1):3–8. doi: 10.1093/oxfordjournals.bmb.a070511. [DOI] [PubMed] [Google Scholar]
- Werdelin O., Wick G., McCluskey R. T. The fate of newly formed lymphocytes migrating from an antigen-stimulated lymph node in rats with allergic adrenalitis. Lab Invest. 1971 Sep;25(3):279–286. [PubMed] [Google Scholar]


