Abstract
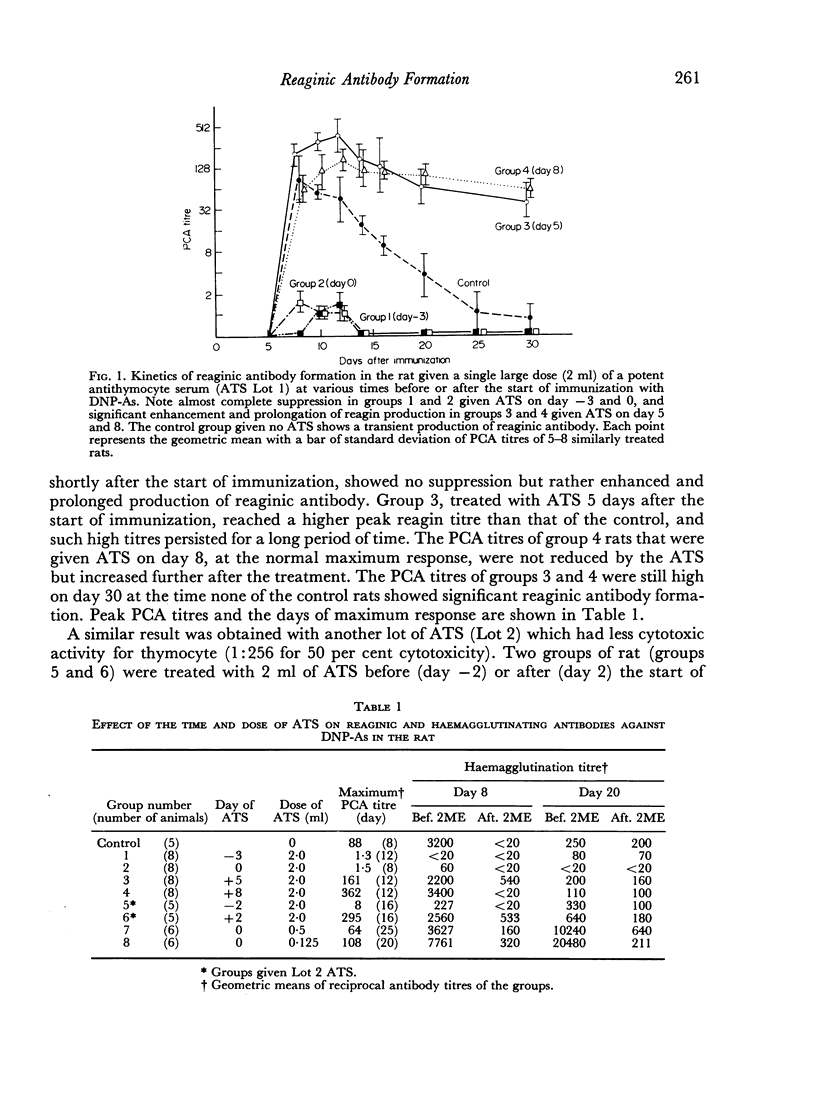
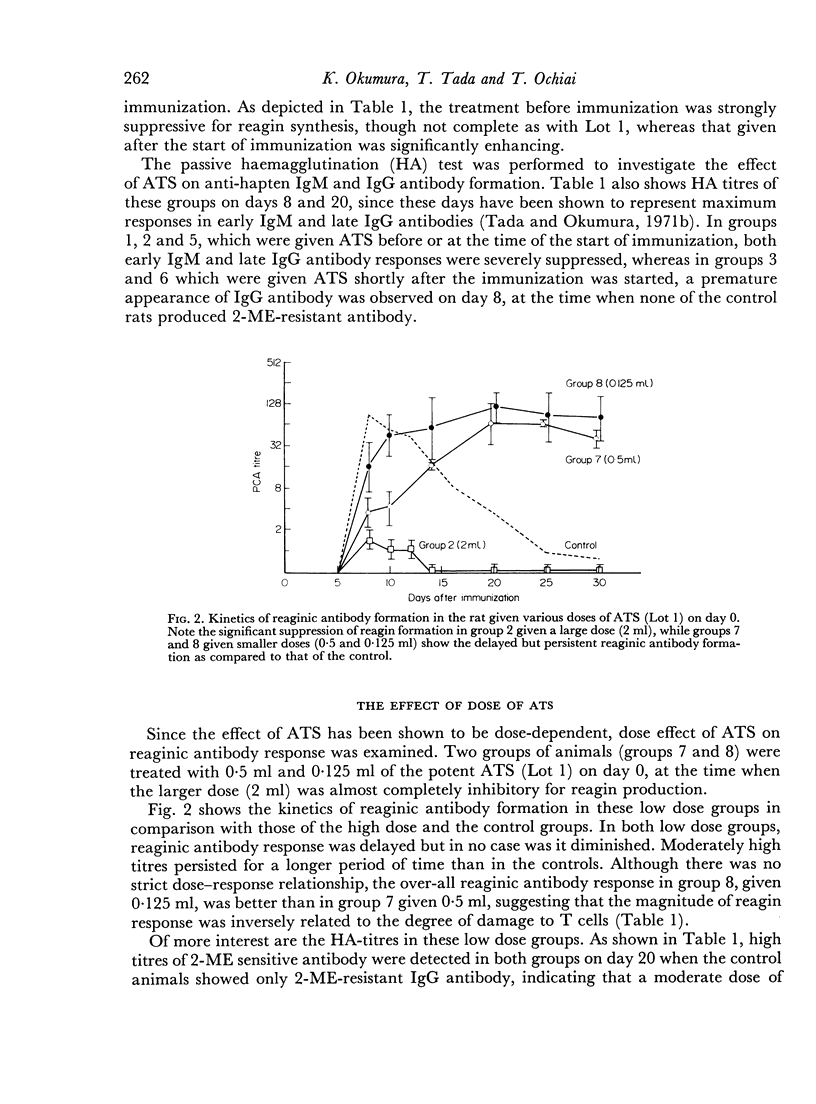
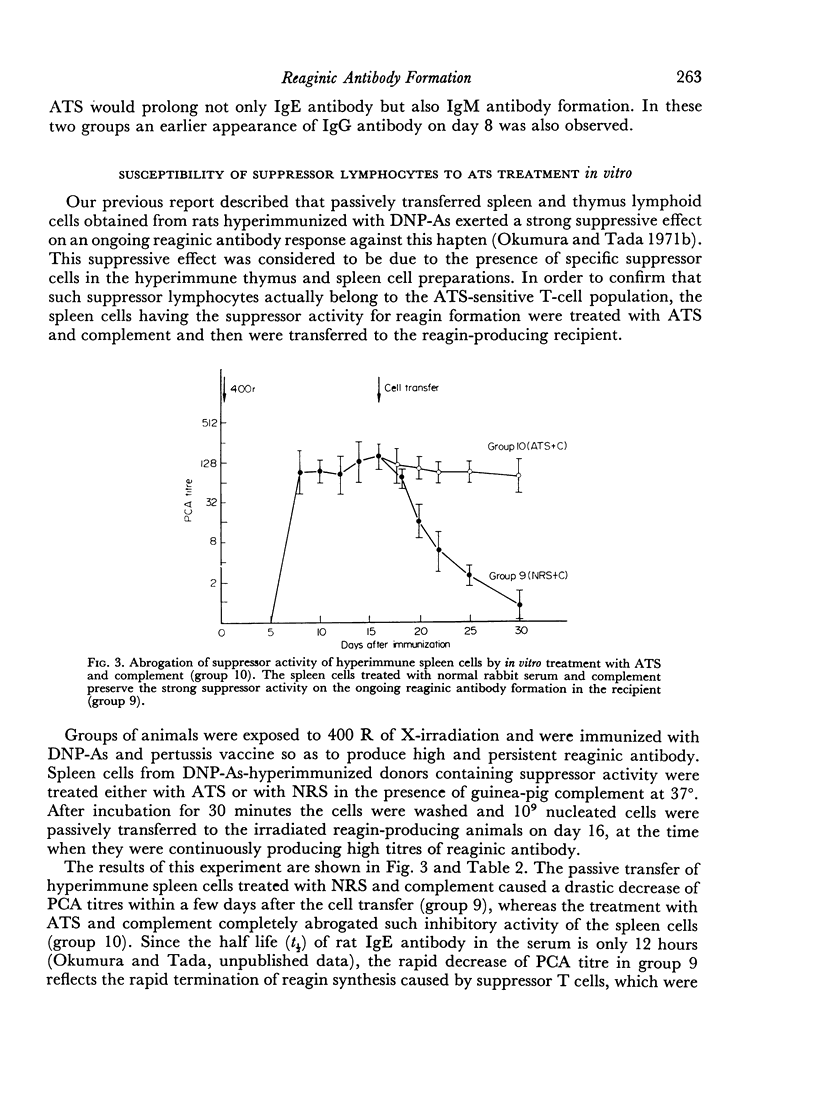
The effect of the time and dose of heterologous antithymocyte serum (ATS) on reaginic antibody formation was studied in the rat. Animals were treated with a single intravenous injection of ATS at various times before or after the primary immunization with dinitrophenylated Ascaris suum extract (DNP-As) and Bordetella pertussis vaccine. If animals were treated with a large lymphopenic dose of ATS shortly before or at the time of immunization, the production of reaginic as well as IgM and IgG antibodies was greatly suppressed, whereas the same treatment if given shortly after the immunization was started significantly enhanced and prolonged the reagin production. On the other hand, smaller doses of ATS even if given at the time of immunization only delayed the reaginic antibody response which also showed a marked prolongation. Furthermore, an unusual sequential production of IgM and IgG antibodies was observed in some of the ATS-treated animals. These results suggest that ATS can inhibit either inductive or regulatory function of the thymus-derived lymphocytes (T cells) depending on the time when it is administered and on the dose of ATS. Some other supporting data indicating that ATS inhibits the T cells specialized in the regulation of antibody formation are also presented.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BINAGHI R. A., BENACERRAF B. THE PRODUCTION OF ANAPHYLACTIC ANTIBODY IN THE RAT. J Immunol. 1964 Jun;92:920–926. [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Barthold D. R., Stashak P. W., Amsbaugh D. F., Prescott B., Baker P. J. Strain differences in the ability of antithymocyte serum (ATS) to enhance the antibody response of inbred mice to type 3 pneumococcal polysaccharide. Cell Immunol. 1973 Feb;6(2):315–323. doi: 10.1016/0008-8749(73)90031-2. [DOI] [PubMed] [Google Scholar]
- Baum J., Liebermann G., Frenkel E. P. The effect of immunologically induced lymphopenia on antibody formation. J Immunol. 1969 Jan;102(1):187–193. [PubMed] [Google Scholar]
- Berenbaum M. C. Time-dependent immunosuppressive effects of anti-thymocyte serum. Nature. 1967 Sep 30;215(5109):1481–1482. doi: 10.1038/2151481a0. [DOI] [PubMed] [Google Scholar]
- James K., Milne I. The effect of anti-lymphocytic antibody on the humoral immune response in different strains of mice. I. The response to bovine serum albumin. Immunology. 1972 Dec;23(6):897–909. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Kind L. S., Ako D. [Induction of skin-sensitizing antibody to horse gamma-globulin by a horse antimouse thymocyte serum]. Transplantation. 1971 May;11(5):489–491. doi: 10.1097/00007890-197105000-00011. [DOI] [PubMed] [Google Scholar]
- Levey R. H., Medawar P. B. Nature and mode of action of antilymphocytic antiserum. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1130–1137. doi: 10.1073/pnas.56.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Medawar P. Review lecture. Immunosuppressive agents, with special reference to antilymphocytic serum. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):155–172. doi: 10.1098/rspb.1969.0086. [DOI] [PubMed] [Google Scholar]
- Ochiai T., Okumura K., Tada T., Iwasa S. Effect of lymphocytosis-promoting factor of Bordetella pertussis on the immune response. I. Suppression of cellular hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1972;43(2):196–206. doi: 10.1159/000230837. [DOI] [PubMed] [Google Scholar]
- Ochiai T., Okumura K., Tada T., Iwasa S. Effect of lymphocytosis-promoting factor of Bordetella pertussis on the immune response. I. Suppression of cellular hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1972;43(2):196–206. doi: 10.1159/000230837. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. 3. Effect of thymectomy and splenectomy. J Immunol. 1971 Apr;106(4):1019–1025. [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- STAVITSKY A. B., ARQUILLA E. R. Micromethods for the study of proteins and antibodies. III. Procedures and applications of hemagglutination and hemagglutination-inhibition reactions with bis-diazotized benzidine and protein-conjugated red blood cells. J Immunol. 1955 Apr;74(4):306–312. [PubMed] [Google Scholar]
- Strannegård O., Belin L. Suppression of reagin synthesis in rabbits by passively administered antibody. Immunology. 1970 May;18(5):775–785. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. V, Cell cooperation in the anti-hapten homocytotropic antibody response. J Immunol. 1971 Oct;107(4):1137–1145. [PubMed] [Google Scholar]
- Tada T., Okumura K., Taniguchi M. Regulation of homocytotropic antibody formation in the rat. VII. Carrier functions in the anti-hapten homocytotropic antibody response. J Immunol. 1972 Jun;108(6):1535–1541. [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Regulation of homocytotropic antibody formation in the rat. IV. Effects of various immunosuppressive drugs. J Immunol. 1971 Aug;107(2):579–585. [PubMed] [Google Scholar]
- Taub T. N. Biological effects of heterologous antilymphocyte serum. Prog Allergy. 1970;14:208–258. [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- White G. J., Holm M. S. Induction of reagin synthesis in the rat with associated and dissociated hemocyanin: effect of antilymphocyte serum. J Immunol. 1973 Feb;110(2):327–334. [PubMed] [Google Scholar]


