Abstract
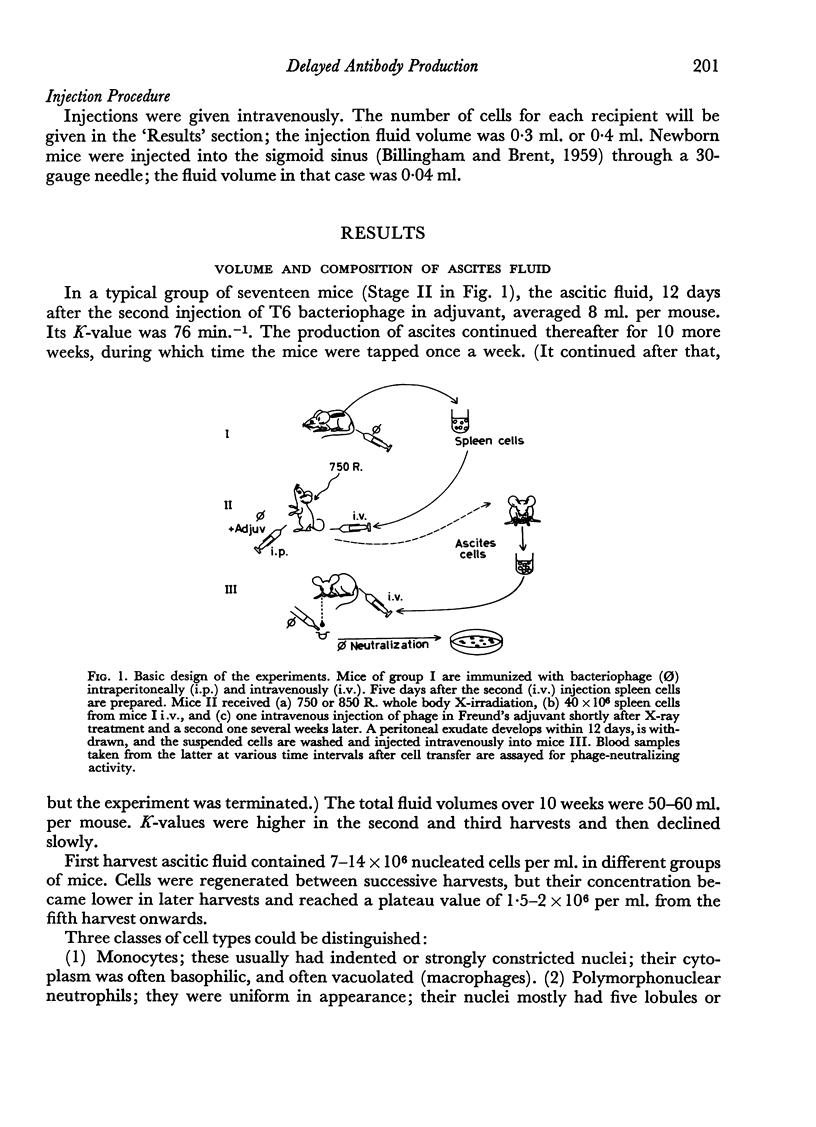
Ascites fluid, rich in bacteriophage-neutralizing antibody, was produced when mice were treated first, with lethal or near-lethal whole body X-radiation; secondly, intravenous injection of spleen cells from donor mice immunized against bacteriophage; and thirdly, with an intraperitoneal injection of bacteriophage in Freund's adjuvant. The `immune ascites cells' were washed and transferred to other mice without further addition of antigen. The production of phage-neutralizing antibody in recipient mice showed the following properties.
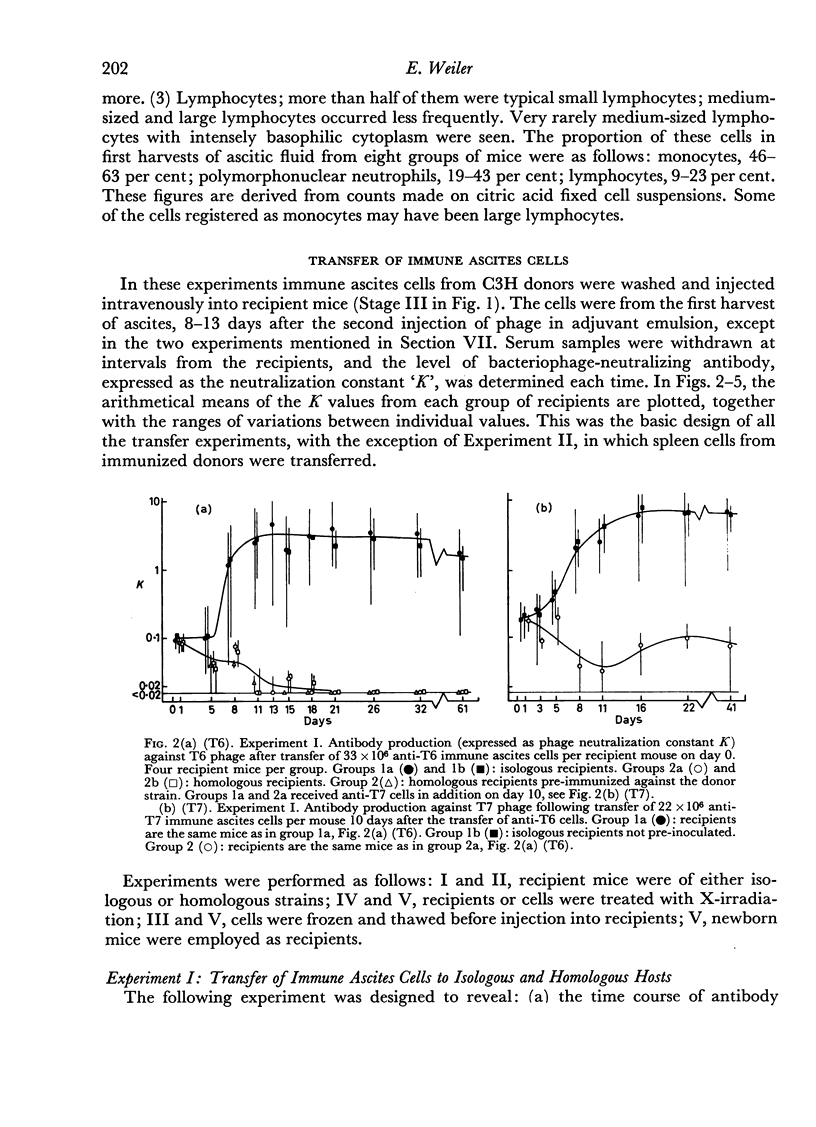
(1) The highest rate of antibody synthesis occurred between the 5th and the 11th day after cell transfer. In contrast, spleen cells similarly transferred gave rise to antibody formation with the maximum rate of synthesis immediately after transfer.
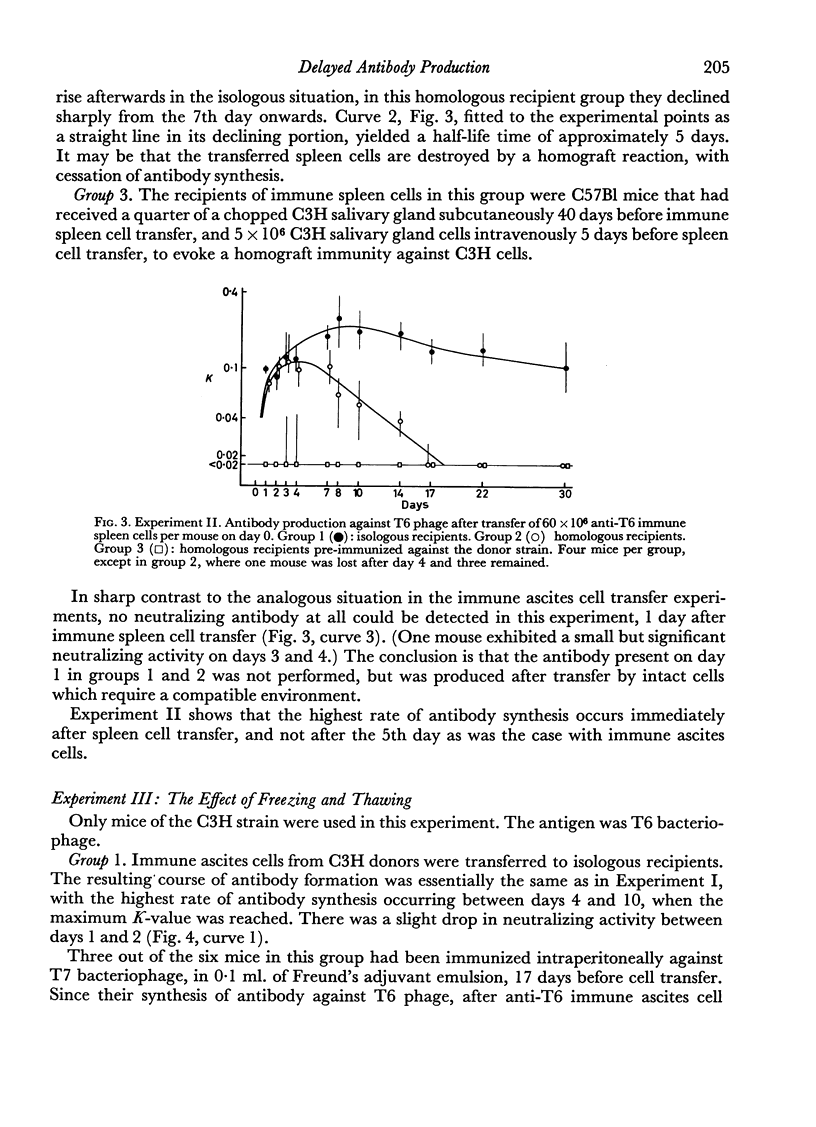
(2) The antibody formation occurred essentially only in isologous recipients, not in homologous ones, whether the latter were pre-immunized against cells of the donor strain or not. With spleen cells, antibody synthesis was not impaired in homologous hosts for about 4 days after transfer, if the hosts were not pre-immunized against the donor strain.
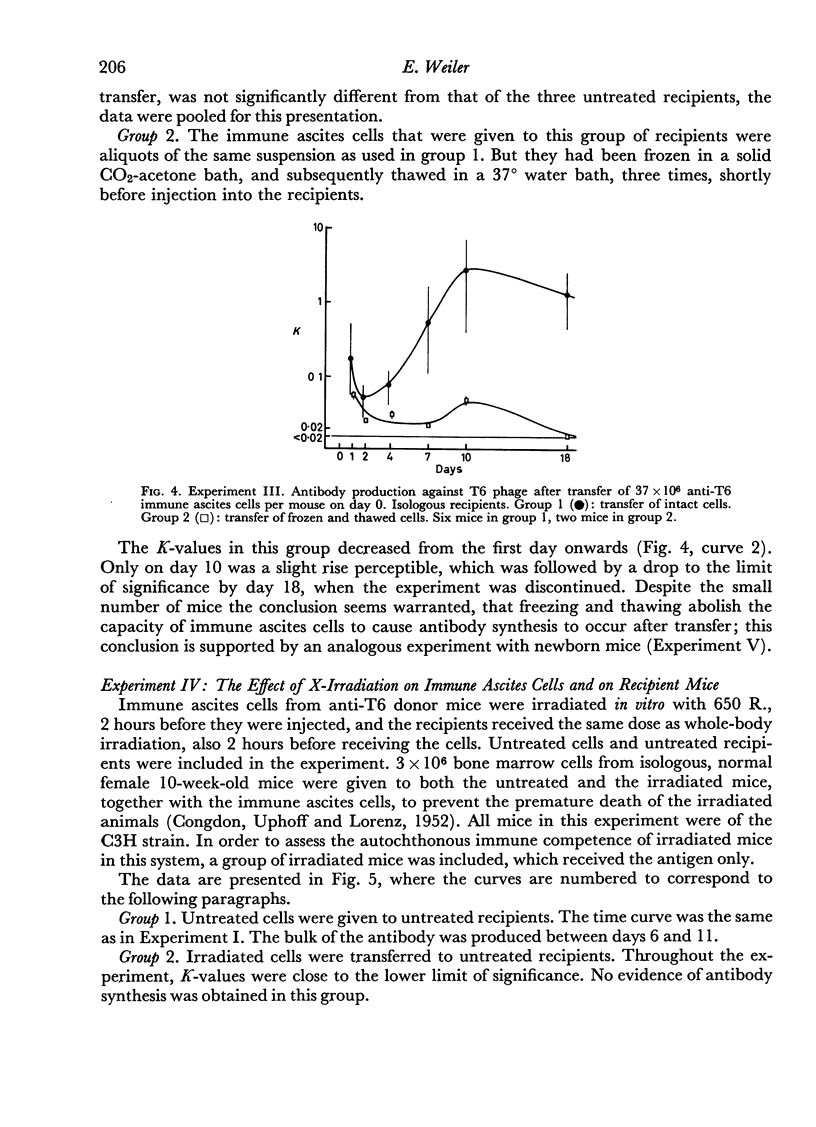
(3) Freezing and thawing of the donor cells prior to injection into the hosts abolished subsequent antibody synthesis.
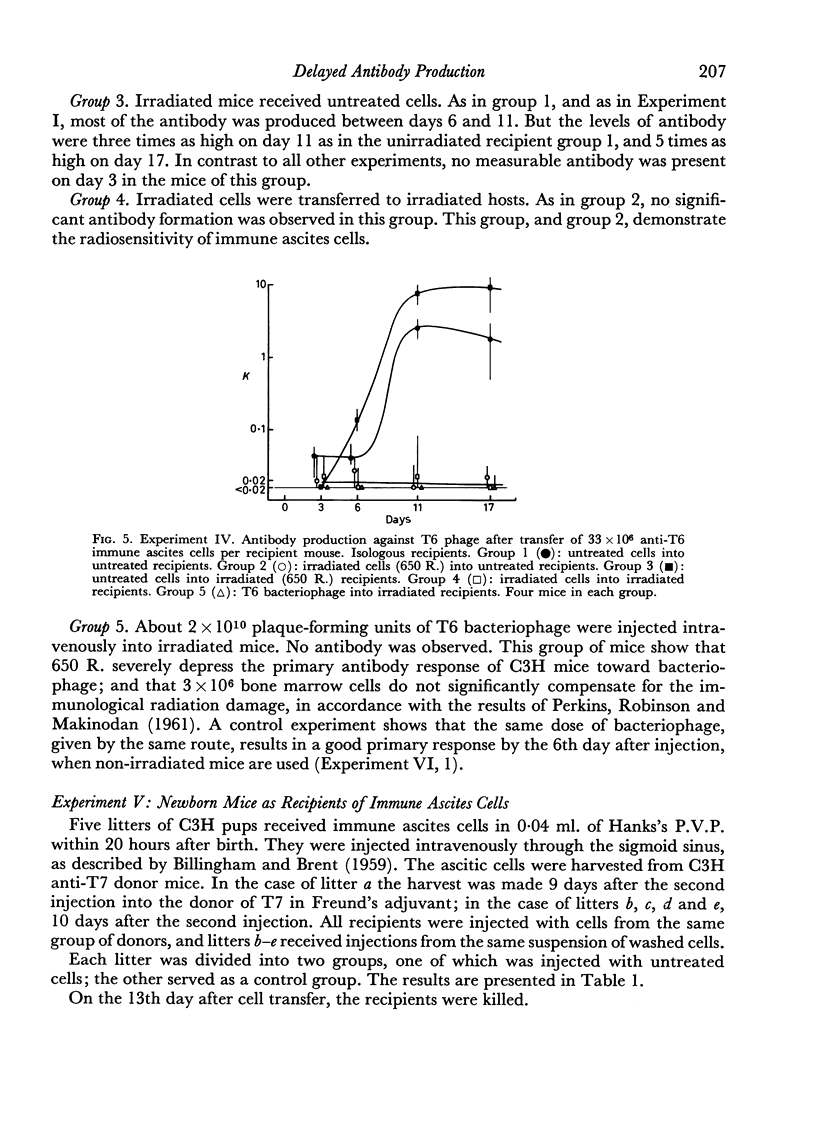
(4) Irradiation of the cells with 650 R. abolished antibody formation after transfer.
(5) Whole-body irradiation of the recipient mice resulted in increased antibody formation.
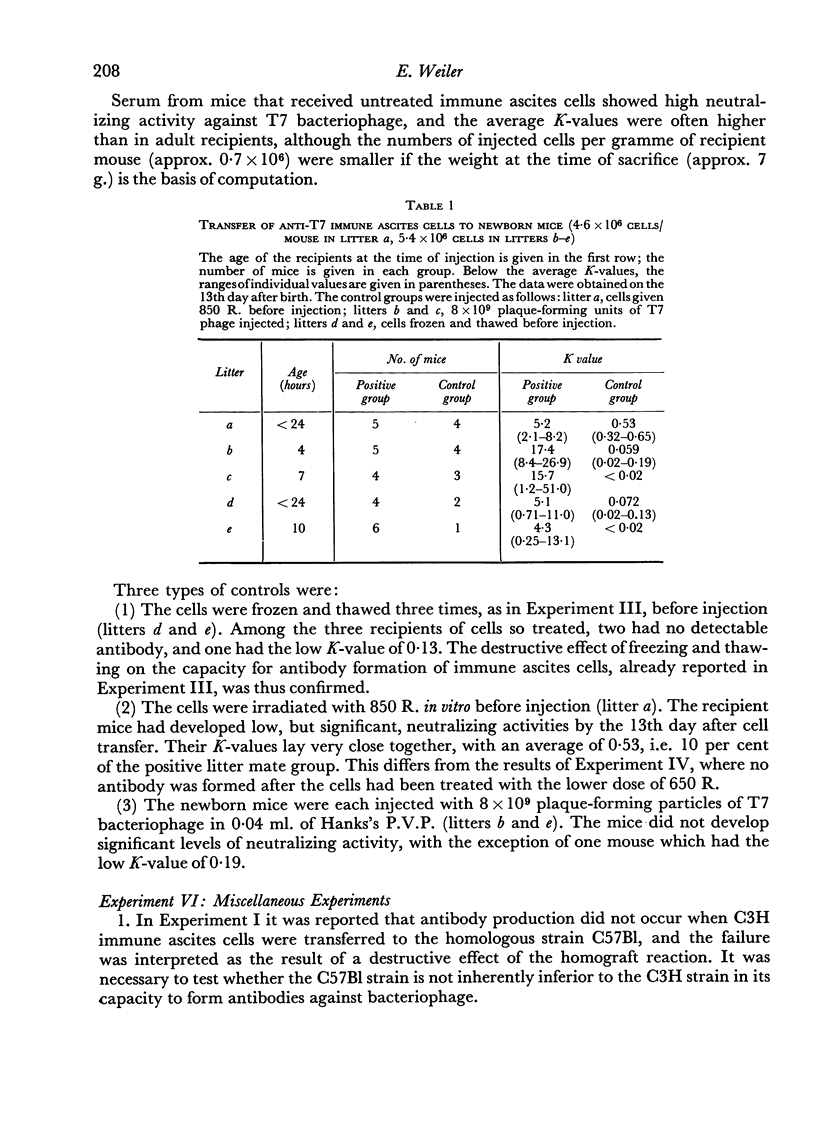
(6) When immune ascites cells were injected into newborn mice, high levels of antibody were found 13 days afterwards.
It is concluded (a) that the population of immune ascites cells carries both the specific information and the stimulus for antibody synthesis, and (b) that the antibody-forming apparatus is not yet present in a functional state at the time of transfer, but develops several days afterwards in the host mice.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., MUNOZ J. Mouse antibody. I. Characterization and properties of antibody in mouse peritoneal fluid. J Immunol. 1961 Oct;87:426–432. [PubMed] [Google Scholar]
- CONGDON C. C., UPHOFF D., LORENZ E. Modification of acute irradiation injury in mice and guinea pigs by injection of bone marrow; a histopathologic study. J Natl Cancer Inst. 1952 Aug;13(1):73–107. [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., WEIGLE W. O., ROBERTS J. C. Comparison of antibody responses associated with the transfer of rabbit lymph-node, peritoneal exudate, and thymus cells. J Immunol. 1957 Jan;78(1):56–62. [PubMed] [Google Scholar]
- DORIA G., GOODMAN J. W., GENGOZIAN N., CONGDON C. C. Immunologic study of antibody-forming cells in mouse radiation chimeras. J Immunol. 1962 Jan;88:20–30. [PubMed] [Google Scholar]
- EPSTEIN H. T. Identification of radiosensitive volume with nucleic acid volume. Nature. 1953 Feb 28;171(4348):394–395. doi: 10.1038/171394a0. [DOI] [PubMed] [Google Scholar]
- FISHMAN M., ADLER F. L. Antibody formation initiated in vitro. II. Antibody synthesis in x-irradiated recipients of diffusion chambers containing nucleic acid derived from macrophages incubated with antigen. J Exp Med. 1963 Apr 1;117:595–602. doi: 10.1084/jem.117.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS S., HARRIS T. N., FARBER M. B. Studies on the transfer of lymph node cells. XII. The effect of anti-rabbit-leucocyte serum on the transfer of antigen-incubated lymph node cells. J Exp Med. 1958 Oct 1;108(4):411–429. doi: 10.1084/jem.108.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS S., HARRIS T. N. Studies on the transfer of lymph node cells. III. Effects of variation in the interval between the injection of antigen into the donor and collection of its lymph node cells. J Exp Med. 1954 Sep 1;100(3):269–287. doi: 10.1084/jem.100.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLMAN R. F., KOHN H. I. The influence of strain on acute x-ray lethality in the mouse. I. LD50 and death rate studies. Radiat Res. 1956 Oct;5(4):309–317. [PubMed] [Google Scholar]
- LEDUC E. H., COONS A. H., CONNOLLY J. M. Studies on antibody production. II. The primary and secondary responses in the popliteal lymph node of the rabbit. J Exp Med. 1955 Jul 1;102(1):61–72. doi: 10.1084/jem.102.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKINODAN T., GENGOZIAN N. Primary antibody response to a distantly related heterologous antigen during maximum depression period after varying doses of x radiation. J Immunol. 1958 Aug;81(2):150–154. [PubMed] [Google Scholar]
- MAKINODAN T., KASTENBAUM M. A., PETERSON W. J. Radiosensitivity of spleen cells from normal and preimmunized mice and its significance to intact animals. J Immunol. 1962 Jan;88:31–37. [PubMed] [Google Scholar]
- MANNICK J. A., EGDAHL R. H. Ribonucleic acid in "transformation" of lymphoid cells. Science. 1962 Sep 21;137(3534):976–977. doi: 10.1126/science.137.3534.976. [DOI] [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS E. H., ROBINSON M. A., MAKINODAN T. Agglutinin response, a function of cell number. J Immunol. 1961 May;86:533–537. [PubMed] [Google Scholar]
- PUCK T. T., MORKOVIN D., MARCUS P. I., CIECIURA S. J. Action of x-rays on mammalian cells. II. Survival curves of cells from normal human tissues. J Exp Med. 1957 Oct 1;106(4):485–500. doi: 10.1084/jem.106.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., EVANS V. J., WALTZ H. K., SHANNON J. E. The measurement of proliferation in tissue cultures by enumeration of cell nuclei. J Natl Cancer Inst. 1951 Feb;11(4):773–795. [PubMed] [Google Scholar]
- Stone S. H. Method for Obtaining Venous Blood from the Orbital Sinus of the Rat or Mouse. Science. 1954 Jan 15;119(3081):100–100. doi: 10.1126/science.119.3081.100. [DOI] [PubMed] [Google Scholar]


