Abstract
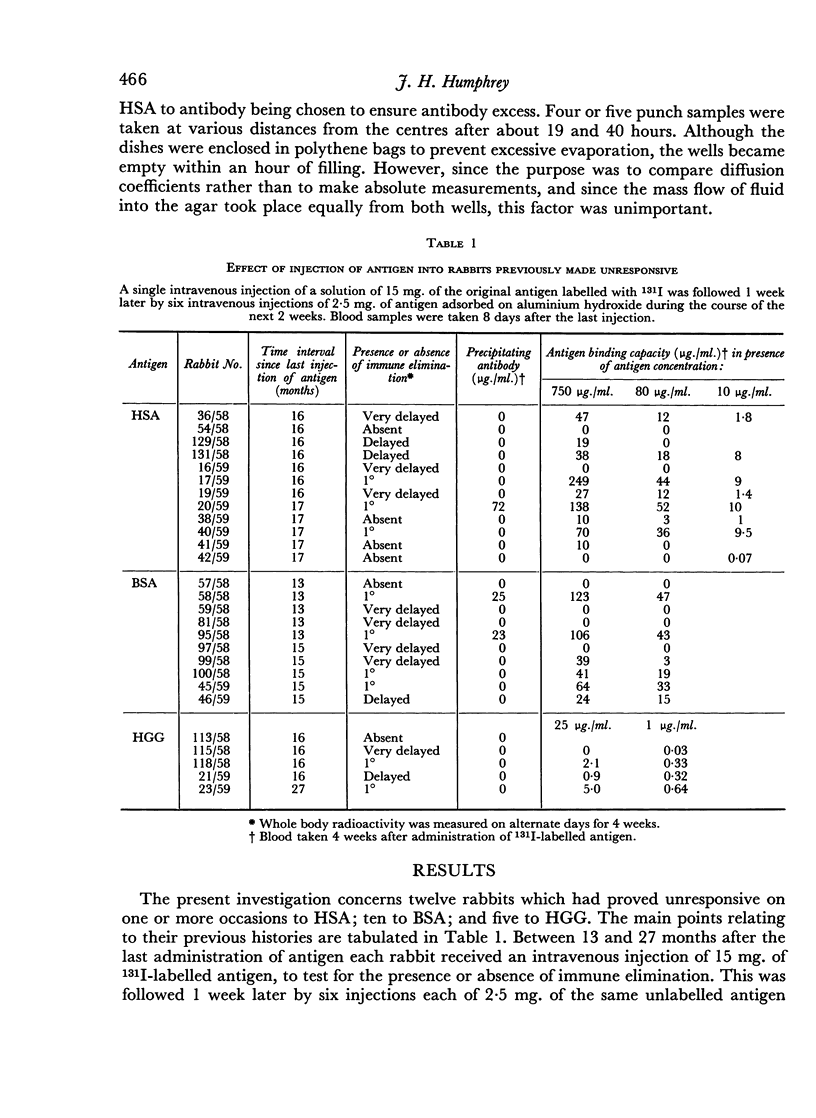
Rabbits made immunologically unresponsive by neonatal administration of HSA, HGG or BSA were given a course of intravenous injections of the respective antigens, adsorbed on alum, after a lapse of 13–27 months since the last administration of antigen. 8/12 responded to HSA, 4/5 to HGG, 9/10 to BSA, as judged by immune elimination of antigen, but this was delayed in onset and slow compared with that in previously untreated rabbits. The antibody formed was small in quantity and usually failed to precipitate with antigen.
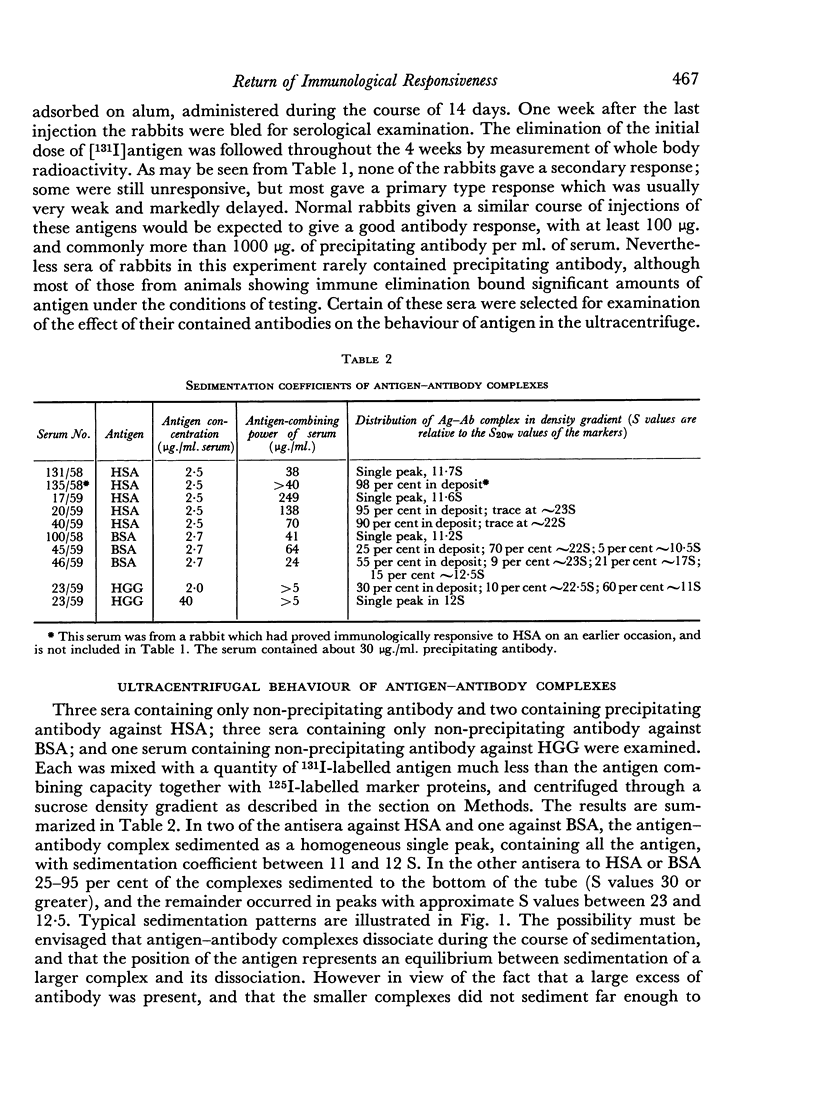
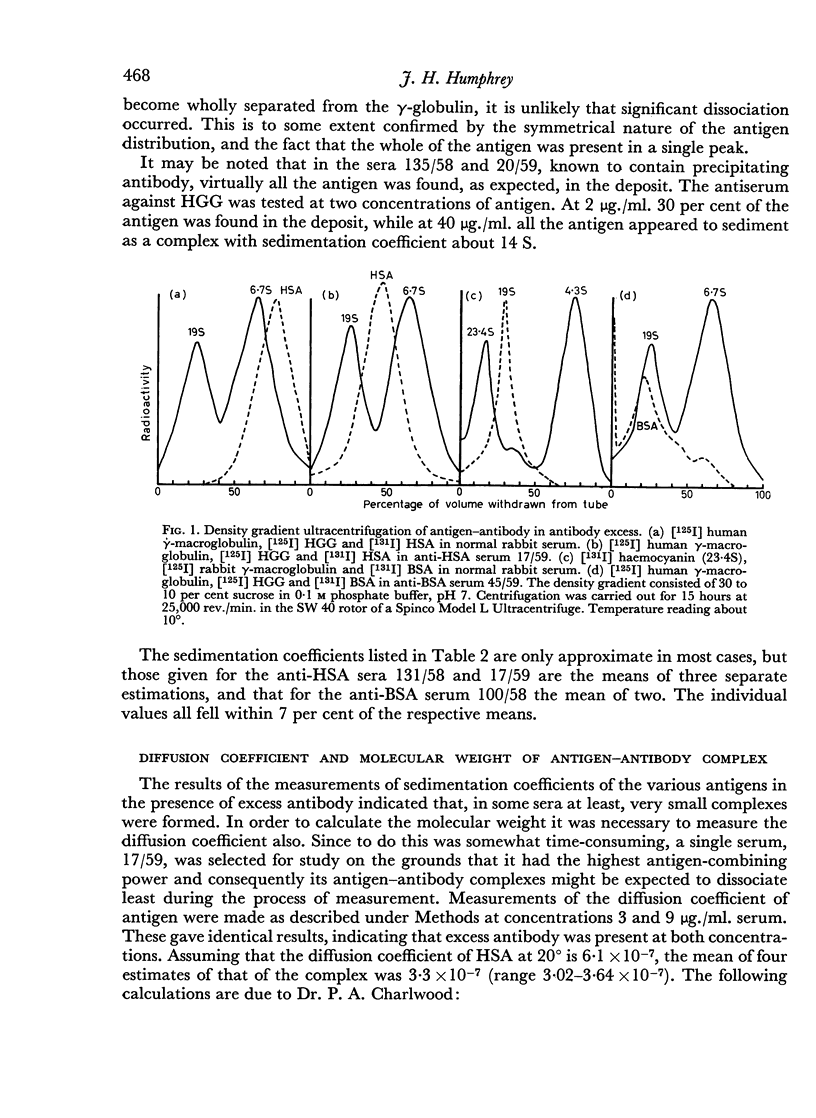
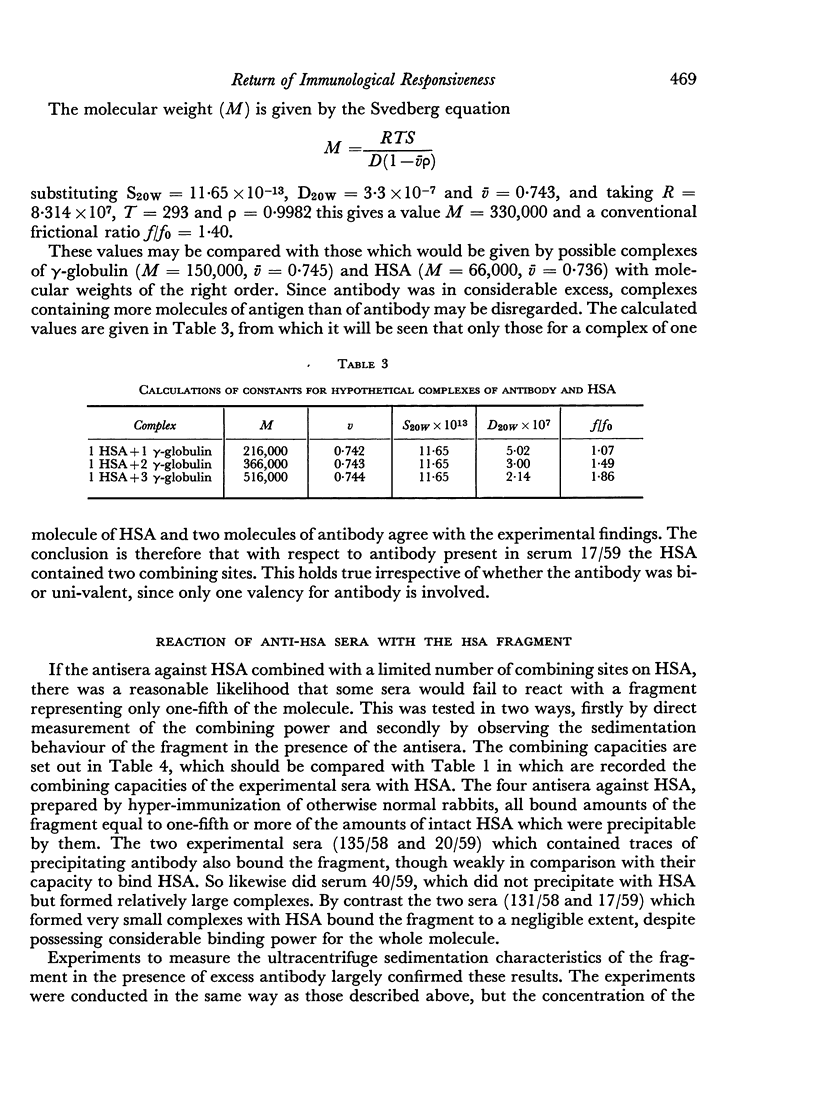
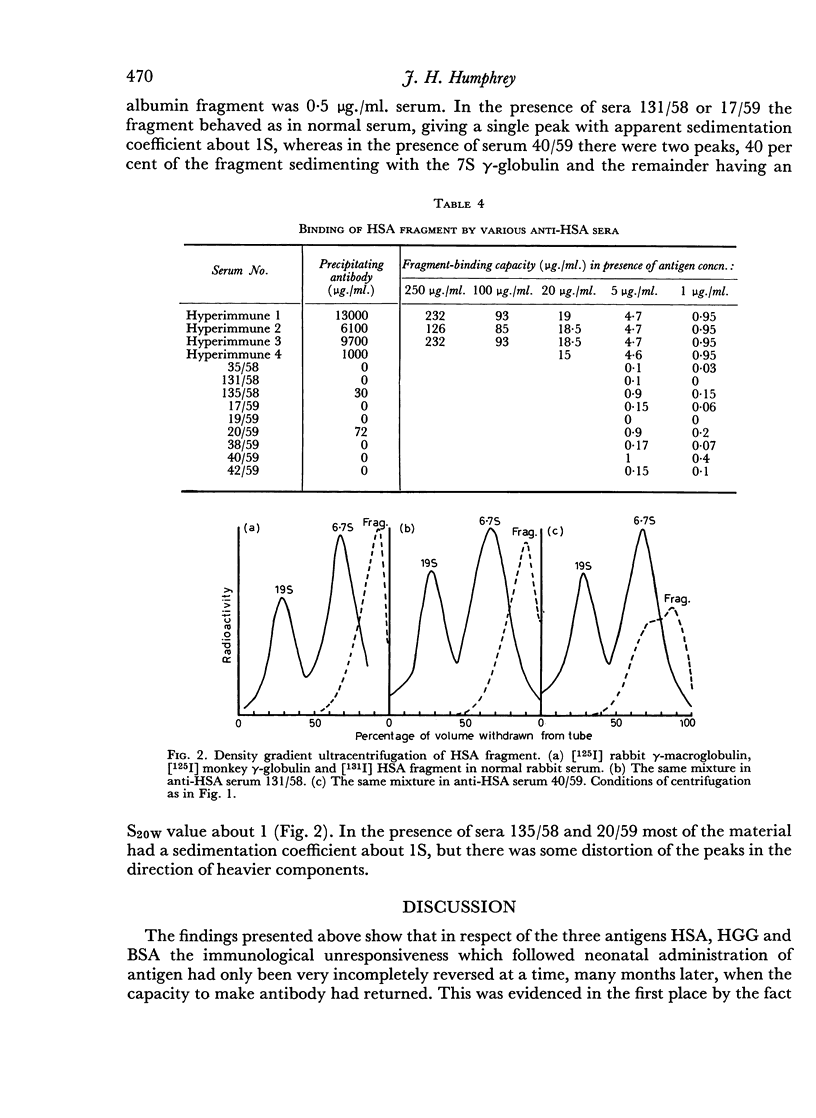
The sedimentation coefficients of 131I-labelled antigens, in the presence of excess antibody, were measured by ultracentrifugation through a sucrose density gradient. These showed that only small complexes were formed in some of the non-precipitating antisera. In one instance the diffusion coefficient of the complex was also measured, by a technique based on diffusion through agar gel. The calculated molecular weight of the complex, 330,000 indicated the presence of only two combining sites on the antigen.
Combination of the anti-HSA sera with an HSA fragment was also measured. Whereas the amount of the fragment bound by ordinary hyperimmune anti-HSA sera was about one-fifth the HSA bound, the amounts bound by the test sera were relatively much less. Some non-precipitating sera failed to bind the fragment, although they bound HSA.
These findings indicate that following neonatally induced immunological unresponsiveness the capacity to respond to antigen returns piecemeal in respect of different parts of the antigenic mosaic, and that it may be severely restricted. The theoretical implications are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., HUMPHREY J. H. A theoretical and experimental analysis of double diffusion precipitin reactions in gels, and its application to characterization of antigens. Immunology. 1960 Jan;3:95–106. [PMC free article] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S. Diverse applications of isotopically labeled insulin. Trans N Y Acad Sci. 1962 Mar;24:487–495. doi: 10.1111/j.2164-0947.1962.tb01423.x. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Applications of radioactively labeled marker proteins in density gradient ultracentrifugation. Anal Biochem. 1963 Mar;5:226–245. doi: 10.1016/0003-2697(63)90120-9. [DOI] [PubMed] [Google Scholar]
- CINADER B., DUBERT J. M. Acquired immune tolerance to human albumin and the response to subsequent injections of diazo human albumin. Br J Exp Pathol. 1955 Oct;36(5):515–529. [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. IMMUNOLOGICAL UNRESPONSIVENESS TO PROTEIN ANTIGENS IN RABBITS. I. THE DURATION OF UNRESPONSIVENESS FOLLOWING A SINGLE INJECTION AT BIRTH. Immunology. 1964 Jul;7:449–461. [PMC free article] [PubMed] [Google Scholar]
- LAPRESLE C., WEBB T. [Study of the degradation of human serum albumin by a rabbit spleen extract. VII. Isolation and properties of a fragment of the albumin molecule]. Ann Inst Pasteur (Paris) 1960 Oct;99:523–532. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]


