Abstract
Three immune globulins in maternal serum and colostrum and newly born calf serum, have been characterized and compared. An examination was made to determine first, which of the maternal serum immune globulins accumulate in the circulation of the calf and secondly, the selectivity of the mammary gland for these proteins compared with the intestinal mucosa of the newly born calf.
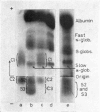
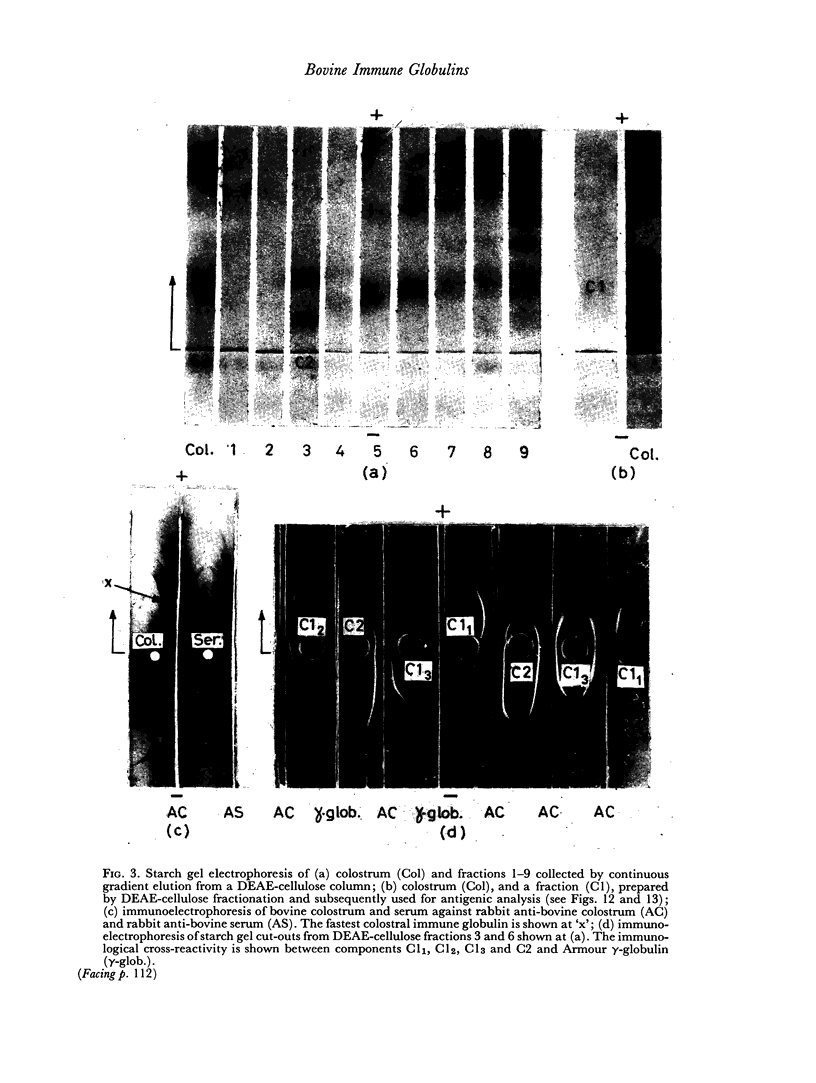
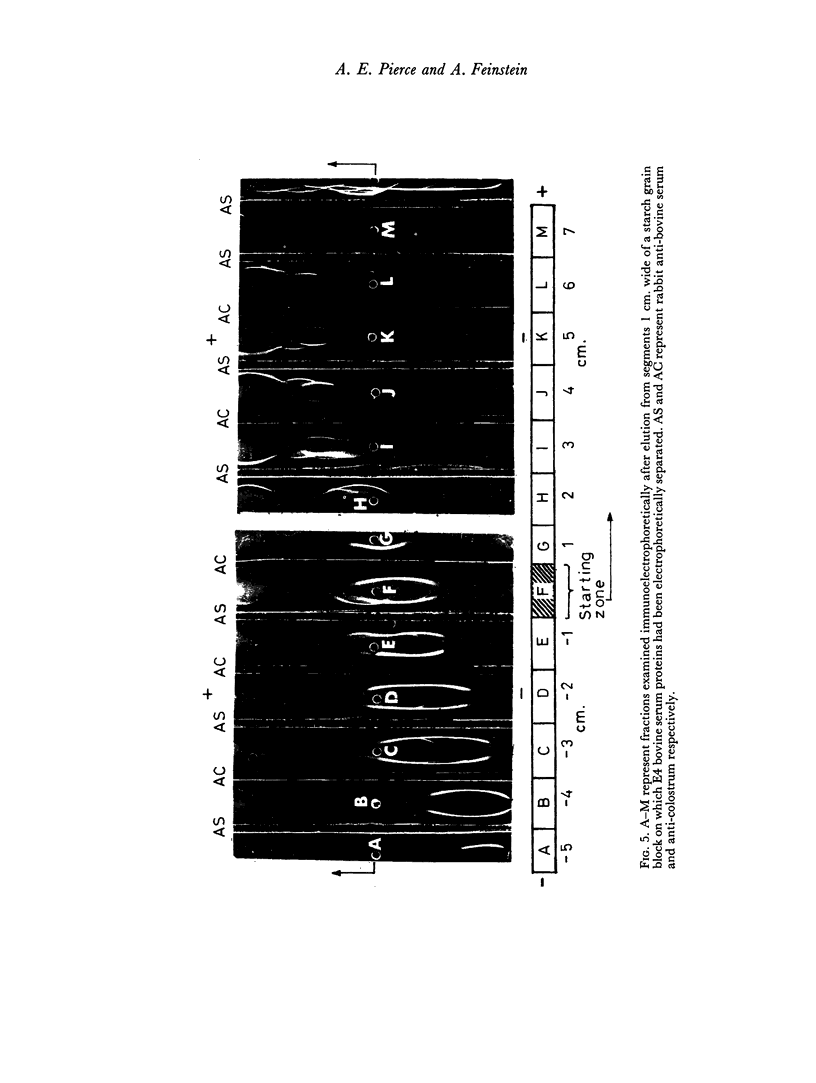
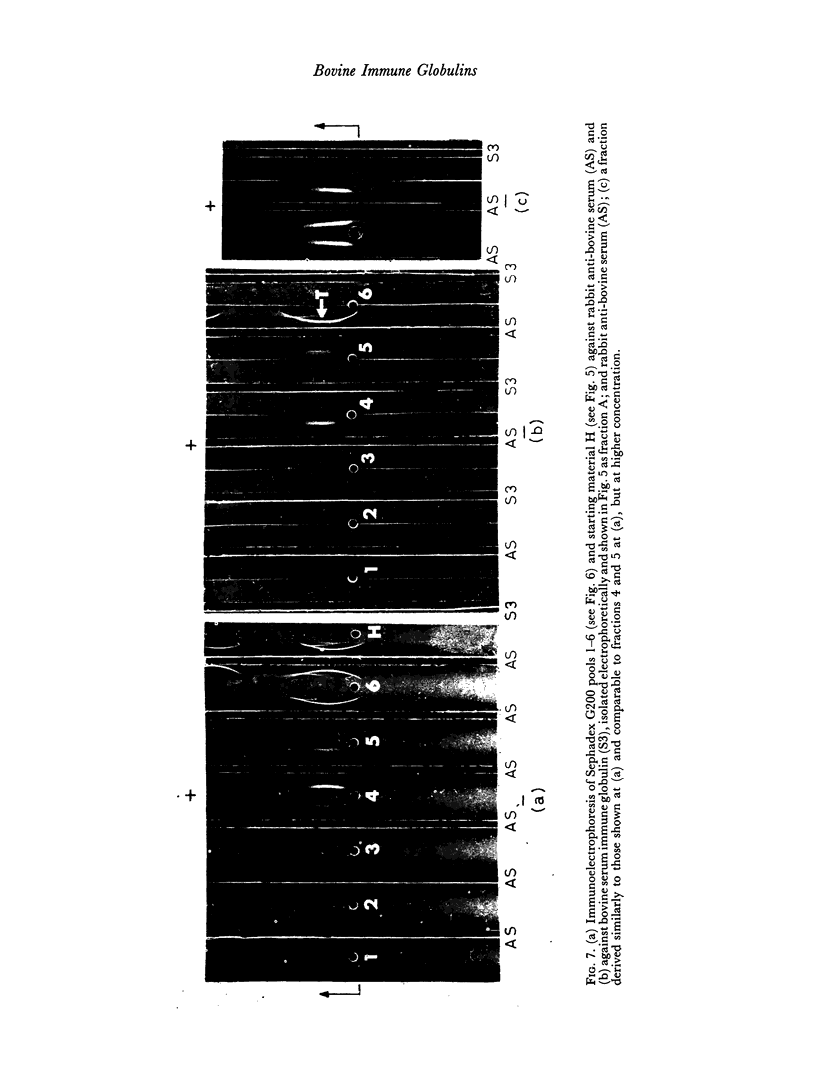
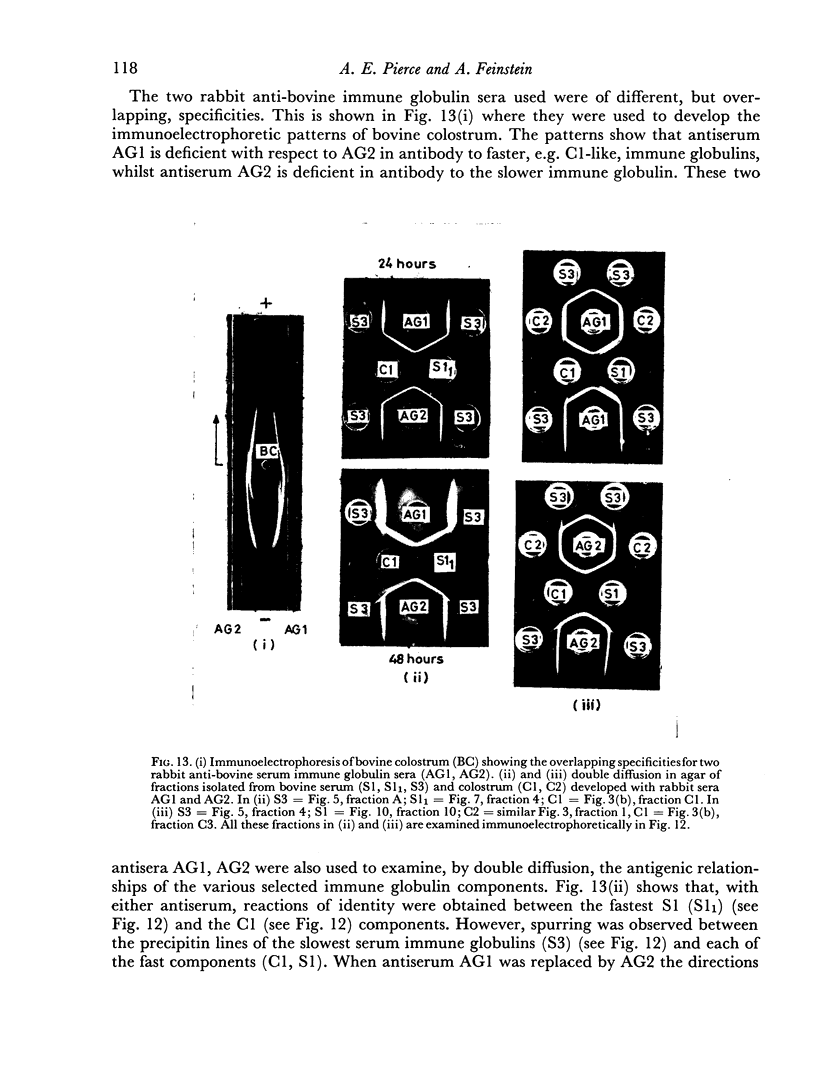
By difference in their electrophoretic mobilities three antigenically related immune globulins were isolated from bovine serum.
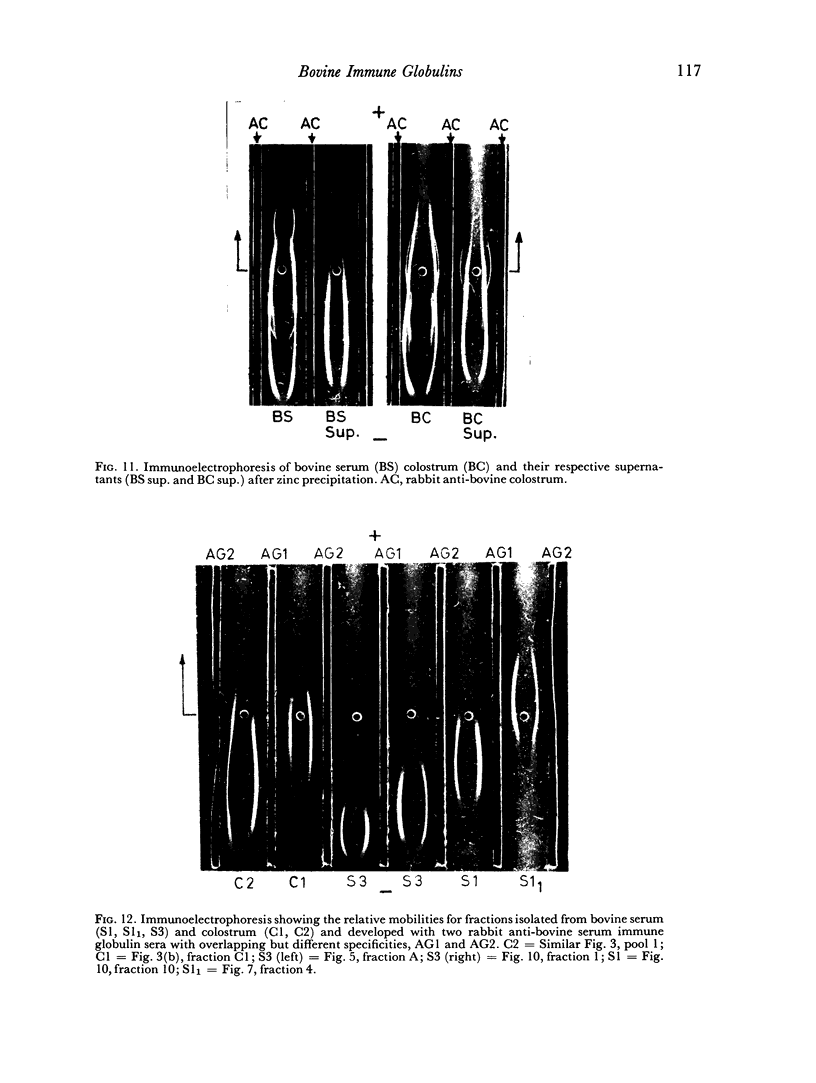
The immune lactoglobulins in bovine colostrum were qualitatively similar to those in serum. However, marked differences were observed between the relative concentrations in serum and colostrum of the three immune globulins.
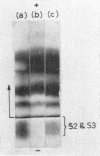
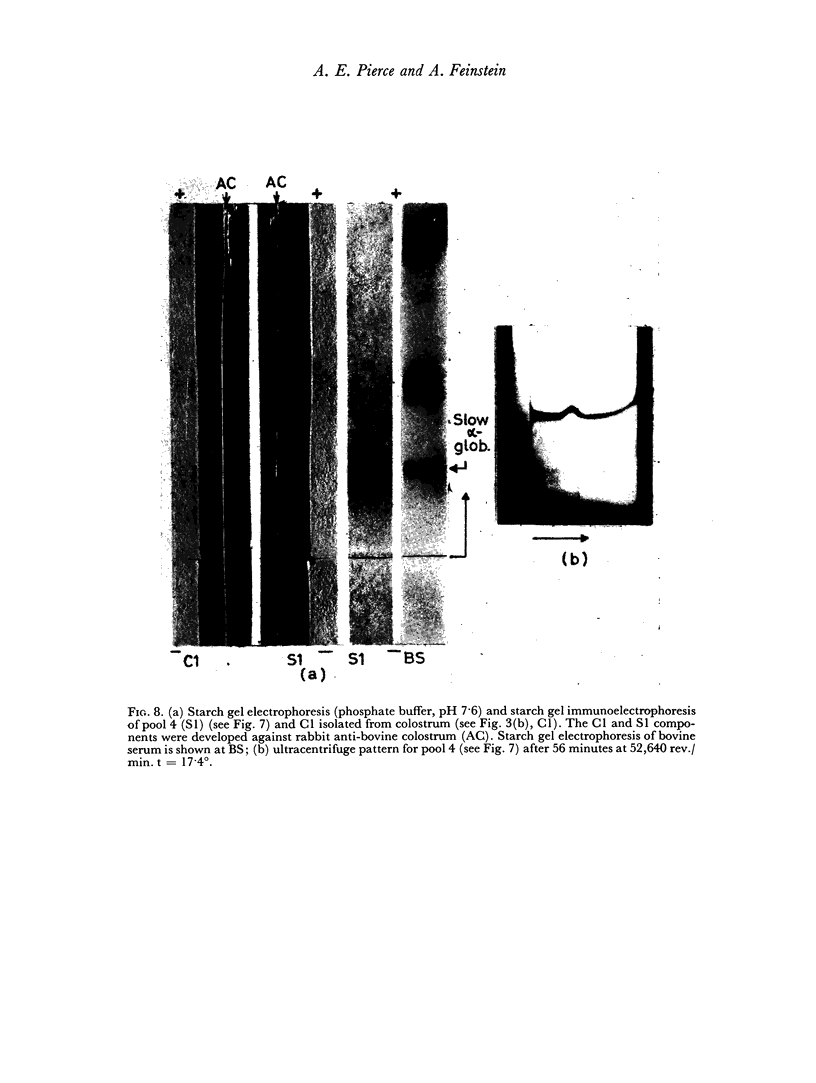
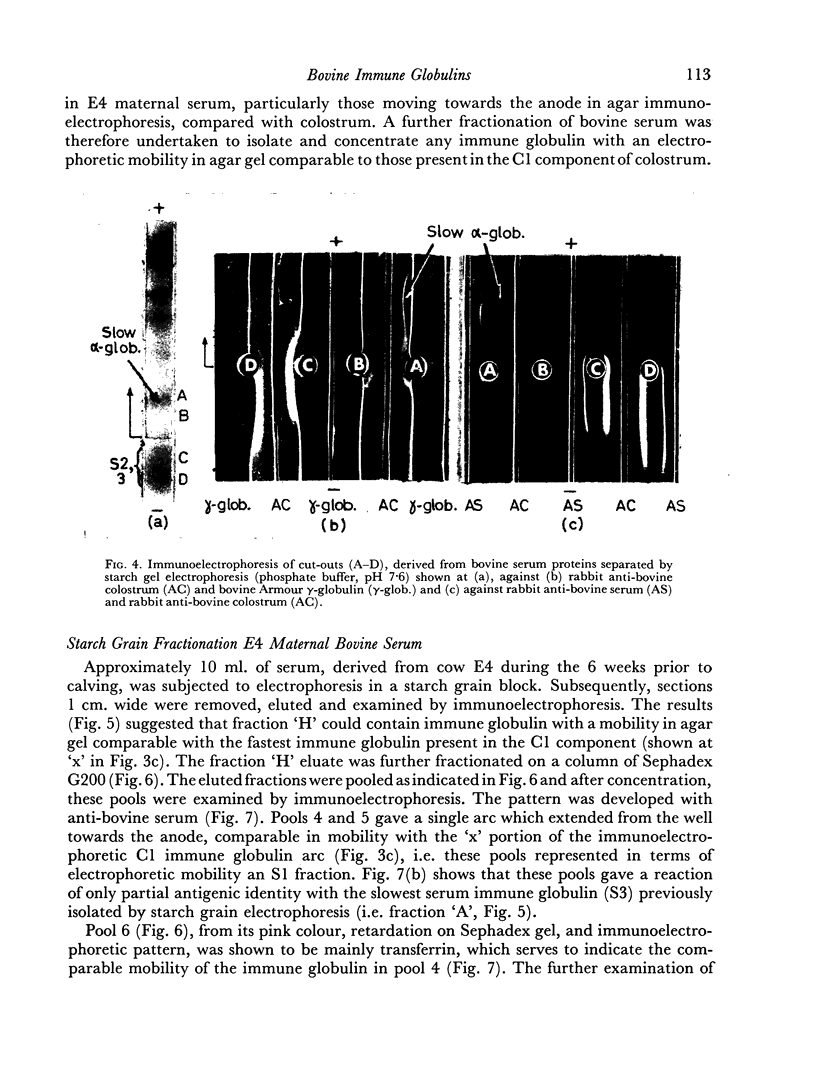
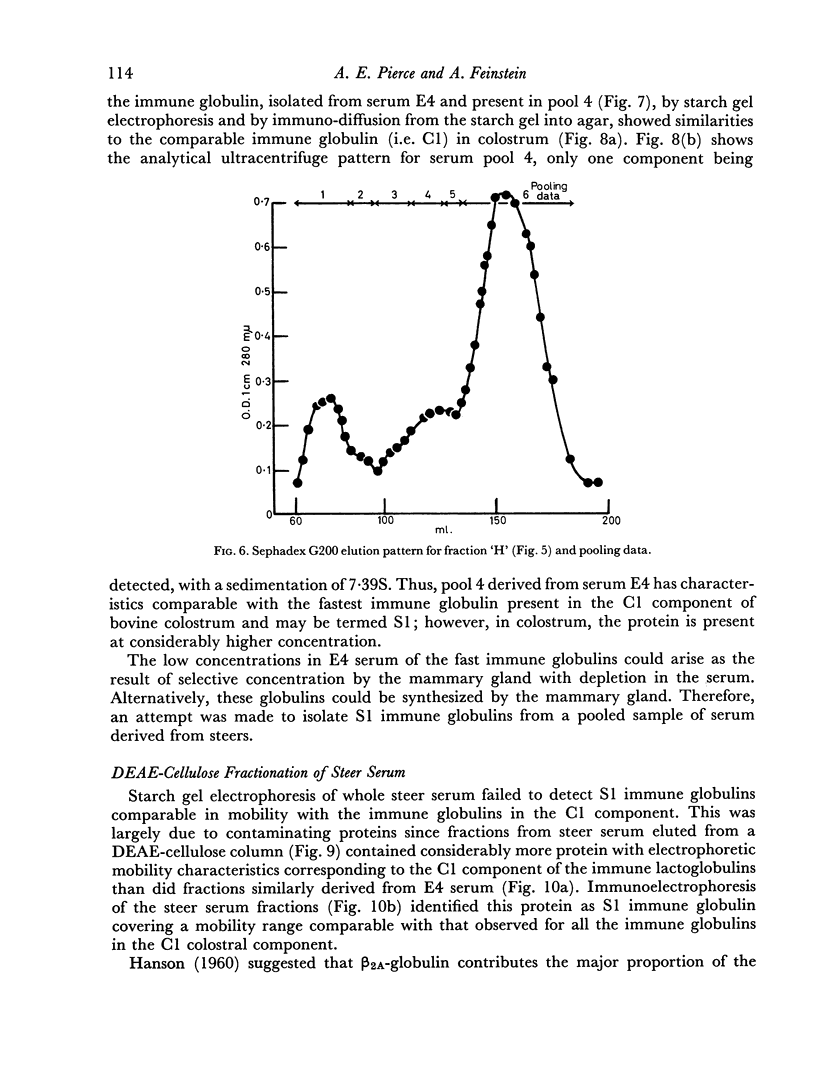
An electrophoretically fast immune globulin (C1), present in colostrum at high concentration, was shown to be antigenically similar to an immune globulin (S1) present in the maternal serum at low concentration.
These findings indicate that the mammary gland showed a highly selective preference for, and hence ability to concentrate in, colostrum, the electrophoretically fastest serum immune globulin.
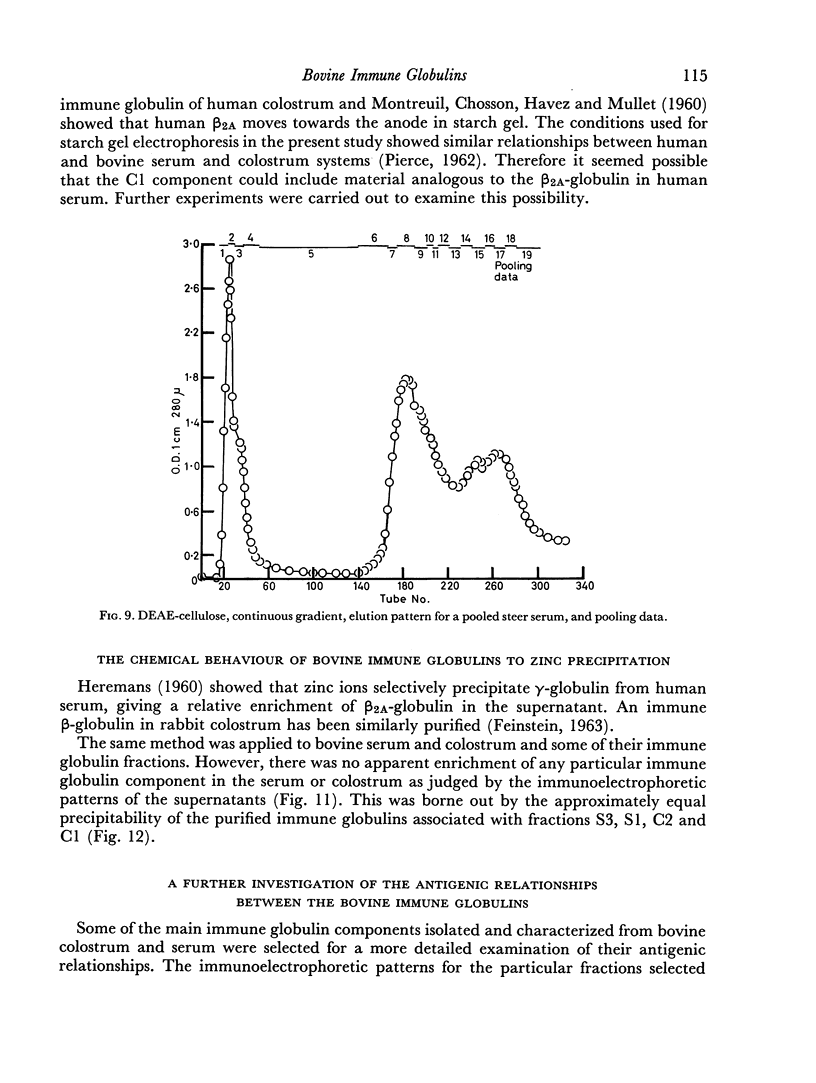
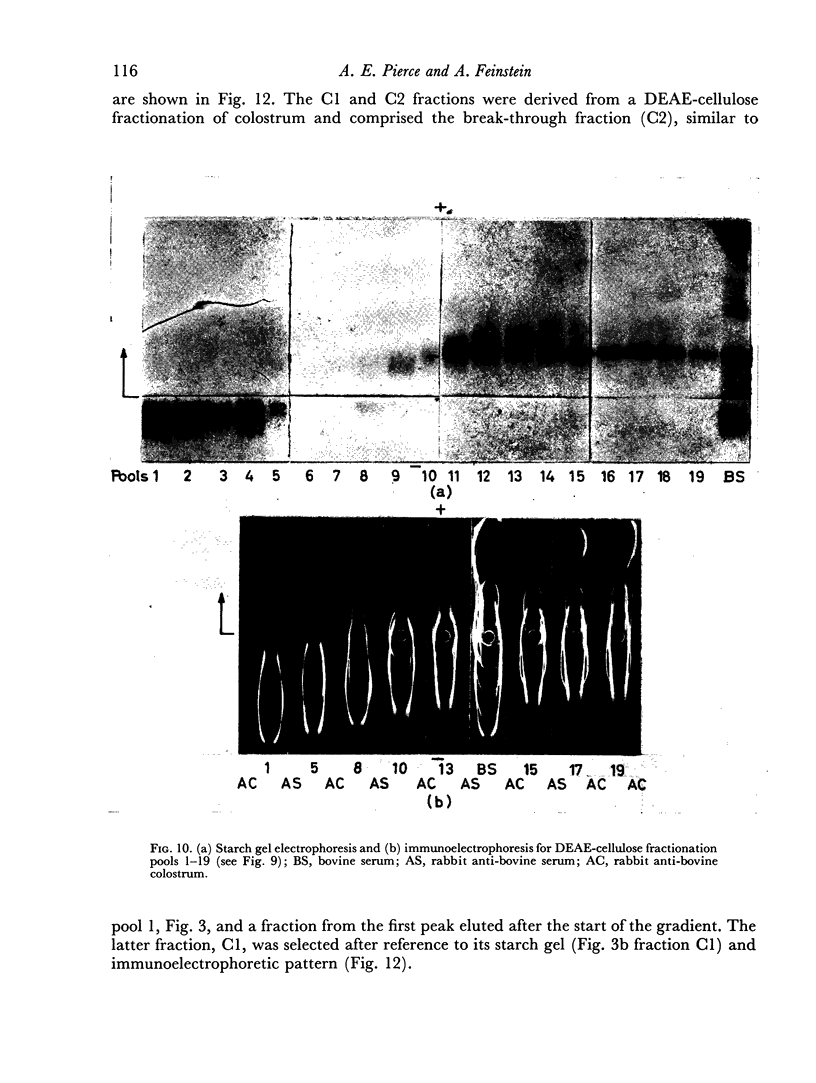
The slowest serum immune globulin and the component with intermediate electrophoretic mobility (S3 and S2 respectively) were both present at high concentration in bovine maternal serum, but were transmitted at different rates into the colostrum, so that the slowest serum immune globulin (S3) was present in the colostrum as a comparatively minor component (C3).
In contrast to the mammary gland, the intestine of the newly born calf (permeable to undegraded protein during the first 24 hours of life) showed no selectivity. Immune globulins showing the three electrophoretic mobilities were absorbed equally readily.
Thus, while the bovine mammary gland showed a highly selective preference for certain electrophoretically different serum proteins, no comparable selectivity was shown by the intestinal mucosa of the newly born calf.
The results emphasize the heterogeneity of bovine immune globulins and show that the calf receives into its circulation from ingested colostrum selected maternal serum immune globulins. This selection of proteins from maternal plasma, for admission to the calf's circulation, occurs within the mammary gland during the formation of colostrum but not during absorption across the calf's intestinal mucosa.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON G. C. Serum protein differences in cattle by starch gel electrophoresis. Nature. 1957 Nov 2;180(4592):917–919. doi: 10.1038/180917a0. [DOI] [PubMed] [Google Scholar]
- ASKONAS B. A., CAMPBELL P. N., HUMPHREY J. H., WORK T. S. The source of antibody globulin in rabbit milk and goat colostrum. Biochem J. 1954 Apr;56(4):597–601. [PMC free article] [PubMed] [Google Scholar]
- BANGHAM D. R., HOBBS K. R., TERRY R. J. Selective placental transfer of serum-proteins in the rhesus. Lancet. 1958 Aug 16;2(7042):351–354. doi: 10.1016/s0140-6736(58)90264-2. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., INGRAM P. L., ROY J. H., SHILLAM K. W., TERRY R. J. The absorption of 1311-labelled serum and colostral proteins from the gut of the young calf. Proc R Soc Lond B Biol Sci. 1958 Dec 4;149(935):184–191. doi: 10.1098/rspb.1958.0061. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R. The transmission of homologous serum proteins to the foetus and to the amniotic fluid in the rhesus monkey. J Physiol. 1960 Sep;153:265–289. doi: 10.1113/jphysiol.1960.sp006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTY I., BRAMBELL F. W., HEMMINGS W. A., OAKLEY C. L. Selection of antitoxins by the foetal membranes of rabbits. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):452–471. doi: 10.1098/rspb.1954.0036. [DOI] [PubMed] [Google Scholar]
- BISWAS E. R. Selective secretion of circulating antibodies in the milk of the rat. Nature. 1961 Dec 2;192:883–884. doi: 10.1038/192883b0. [DOI] [PubMed] [Google Scholar]
- BLAKEMORE F., GARNER R. J. The maternal transference of antibodies in the bovine. J Comp Pathol. 1956 Oct;66(4):287–289. doi: 10.1016/s0368-1742(56)80030-1. [DOI] [PubMed] [Google Scholar]
- BRAMBELL F. W. R., HEMMINGS W. A., HENDERSON M., ROWLANDS W. T. The selective admission of antibodies to the foetus by the yolk-sac splanchnopleur in rabbits. Proc R Soc Lond B Biol Sci. 1950 Jul 24;137(887):239–252. doi: 10.1098/rspb.1950.0032. [DOI] [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., OAKLEY C. L., PORTER R. R. The relative transmission of the fractions of papain hydrolyzed homologous gamma-globulin from the uterine cavity to the foetal circulation in the rabbit. Proc R Soc Lond B Biol Sci. 1960 Mar 1;151:478–482. doi: 10.1098/rspb.1960.0011. [DOI] [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., OAKLEY C. L. The relative transmission of natural and pepsin-refined homologous antitoxin from the uterine cavity to the foetal circulation in the rabbit. Proc R Soc Lond B Biol Sci. 1959 Apr 21;150(940):312–317. doi: 10.1098/rspb.1959.0024. [DOI] [PubMed] [Google Scholar]
- CINADER B., WEITZ B. The interaction of tetanus toxin and antitoxin. J Hyg (Lond) 1953 Sep;51(3):293–310. doi: 10.1017/s0022172400015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., WEIGLE W. O., VAZQUEZ J. J. Metabolism and mammary secretion of serum proteins in the cow. Lab Invest. 1961 Mar-Apr;10:216–237. [PubMed] [Google Scholar]
- FAHEY J. L., McCOY P. F., GOULIAN M. Chromatography of serum proteins in normal and pathologic sera: the distribution of protein-bound carbohydrate and cholesterol, siderophilin, thyroxin-binding protein, B12-binding protein, alkaline and acid phosphatases, radio-iodinated albumin and myeloma proteins. J Clin Invest. 1958 Feb;37(2):272–284. doi: 10.1172/JCI103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINSTEIN A. CHARACTER AND ALLOTYPY OF AN IMMUNE GLOBULIN IN RABBIT COLOSTRUM. Nature. 1963 Sep 21;199:1197–1199. doi: 10.1038/1991197b0. [DOI] [PubMed] [Google Scholar]
- FELDMAN J. D. Fine structure of the cow's udder during gestation and lactation. Lab Invest. 1961 Mar-Apr;10:238–255. [PubMed] [Google Scholar]
- GARNER R. J., CRAWLEY W. Further observations on the maternal transference of antibodies in the bovine. J Comp Pathol. 1958 Jan;68(1):112–114. doi: 10.1016/s0368-1742(58)80012-0. [DOI] [PubMed] [Google Scholar]
- GUGLER E., BOKELMANN G., DATWYLER A., VON MURALT G. Uber immunoelektrophoretische Untersuchungen an Frauenmilchproteinen. Schweiz Med Wochenschr. 1958 Dec 13;88(50):1264–1267. [PubMed] [Google Scholar]
- HALLIDAY R. The absorption of antibodies from immune sera by the gut of the young rat. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):408–413. doi: 10.1098/rspb.1955.0020. [DOI] [PubMed] [Google Scholar]
- HANSON L. A. Comparative immunological studies of the immune globulins of human milk and of blood serum. Int Arch Allergy Appl Immunol. 1961;18:241–267. doi: 10.1159/000229177. [DOI] [PubMed] [Google Scholar]
- HANSON L. A. The serological relatonship between human milk and blood plasma. Int Arch Allergy Appl Immunol. 1960;17:45–69. doi: 10.1159/000229110. [DOI] [PubMed] [Google Scholar]
- HARTLEY P. The effect of peptic digestion on the properties of diphtheria antitoxin. Proc R Soc Lond B Biol Sci. 1951 Oct 30;138(893):499–513. doi: 10.1098/rspb.1951.0037. [DOI] [PubMed] [Google Scholar]
- HEMMINGS W. A., OAKLEY C. L. Protein selection in the yolk-sac splanchnopleur of the rabbit: the fate of globulin injected into the foetal circulation. Proc R Soc Lond B Biol Sci. 1957 Jun 25;146(925):573–579. doi: 10.1098/rspb.1957.0031. [DOI] [PubMed] [Google Scholar]
- HEMMINGS W. A. Protein selection in the yolk-sac splanchnopleur of the rabbit: the distribution of isotope following injection of 131I-labelled serum globulin into the uterine cavity. Proc R Soc Lond B Biol Sci. 1956 May 29;145(919):186–195. doi: 10.1098/rspb.1956.0026. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., PIERCE A. E. Ultracentrifugal and electrophoretic studies on neonatal calf sera and maternal colostrum. J Hyg (Lond) 1959 Sep;57:309–320. doi: 10.1017/s0022172400020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARTE H. Uber das Vorkommen von Serumproteinen in der Frauenmilch. Monatsschr Kinderheilkd. 1959 May;107(5):265–267. [PubMed] [Google Scholar]
- LARSON B. L., GILLESPIE D. C. Origin of the major specific proteins in milk. J Biol Chem. 1957 Aug;227(2):565–573. [PubMed] [Google Scholar]
- LUNSFORD L., Jr, DEUTSCH H. F. Human milk whey proteins. Proc Soc Exp Biol Med. 1957 Dec;96(3):742–744. doi: 10.3181/00379727-96-23595. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., CHOSSON A., HAVEZ R., MULLET S. [Isolation of beta2A-globulins from human milk]. C R Seances Soc Biol Fil. 1960;154:732–736. [PubMed] [Google Scholar]
- OSSERMAN E. F. A modified technique of immunoelectrophoresis facilitating the identification of specific precipitin arcs. J Immunol. 1960 Jan;84:93–97. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- PATTERSON R., YOUNGNER J. S., WEIGLE W. O., DIXON F. J. The metabolism of serum proteins in the hen and chick and secretion of serum proteins by the ovary of the hen. J Gen Physiol. 1962 Jan;45:501–513. doi: 10.1085/jgp.45.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS F. T., YETTS R., GAISFORD W. Serological response of infants to poliomyelitis vaccine. Br Med J. 1958 Jul 12;2(5088):68–71. doi: 10.1136/bmj.2.5088.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE A. E. Electrophoretic and immunological studies on sera from calves from birth to weaning. I. Electrophoretic studies. J Hyg (Lond) 1955 Sep;53(3):247–260. doi: 10.1017/s0022172400000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE A. E. Further studies on proteinuria in the new-born calf. J Physiol. 1961 Apr;156:136–149. doi: 10.1113/jphysiol.1961.sp006664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE A. E., RISDALL P. C., SHAW B. ABSORPTION OF ORALLY ADMINISTERED INSULIN BY THE NEWLY BORN CALF. J Physiol. 1964 Jun;171:203–215. doi: 10.1113/jphysiol.1964.sp007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE A. E. Studies on the proteinuria of the new-born calf. J Physiol. 1959 Oct;148:469–488. doi: 10.1113/jphysiol.1959.sp006301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]