Abstract
Human polymorphs have been found to ingest a wide variety of particles without the aid of serum, indicating that the cells possess a serum-independent mechanism of phagocytosis. Polymorphs have also been shown to possess a different and complementary mechanism of phagocytosis which depends on the presence of serum and serum components in the medium.
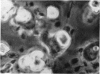
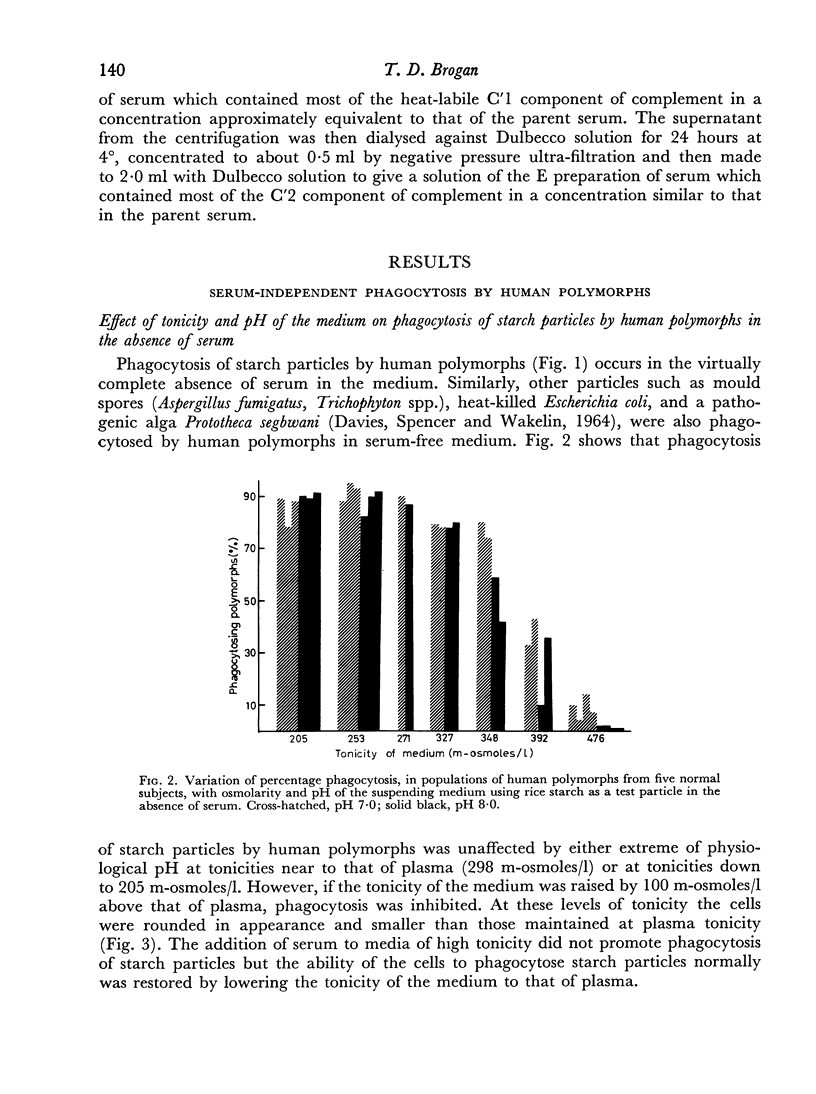
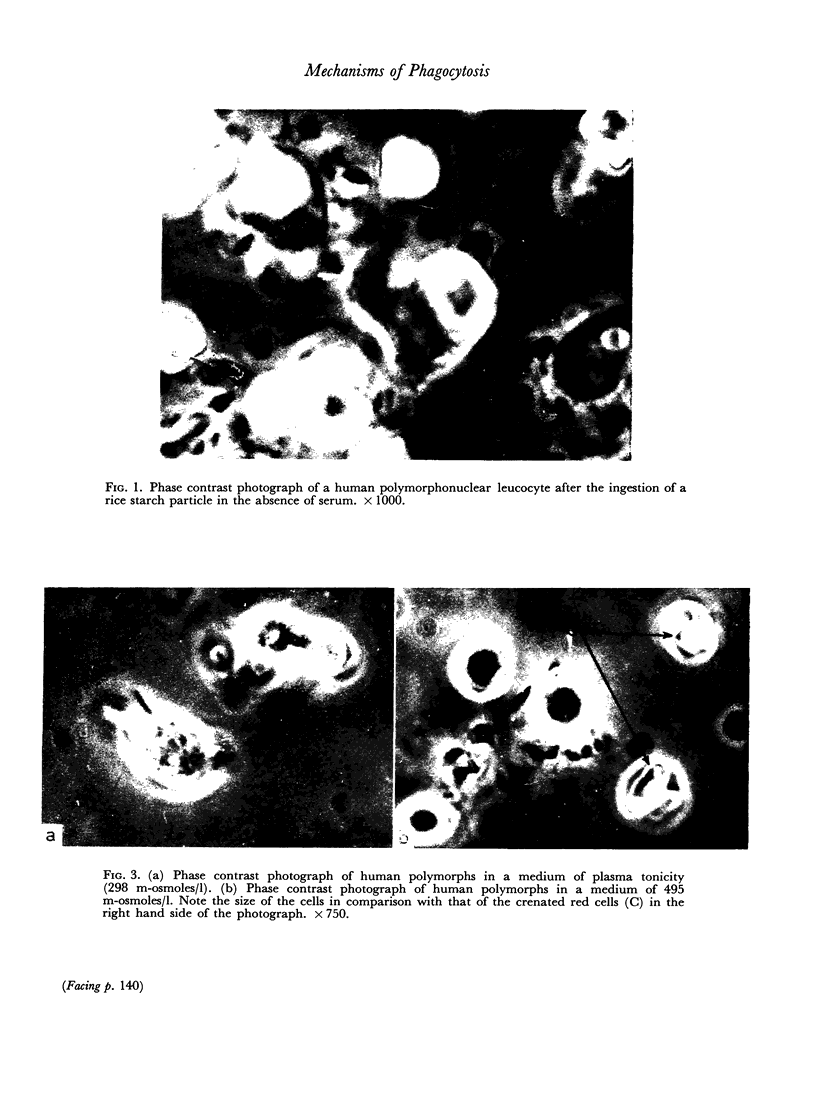
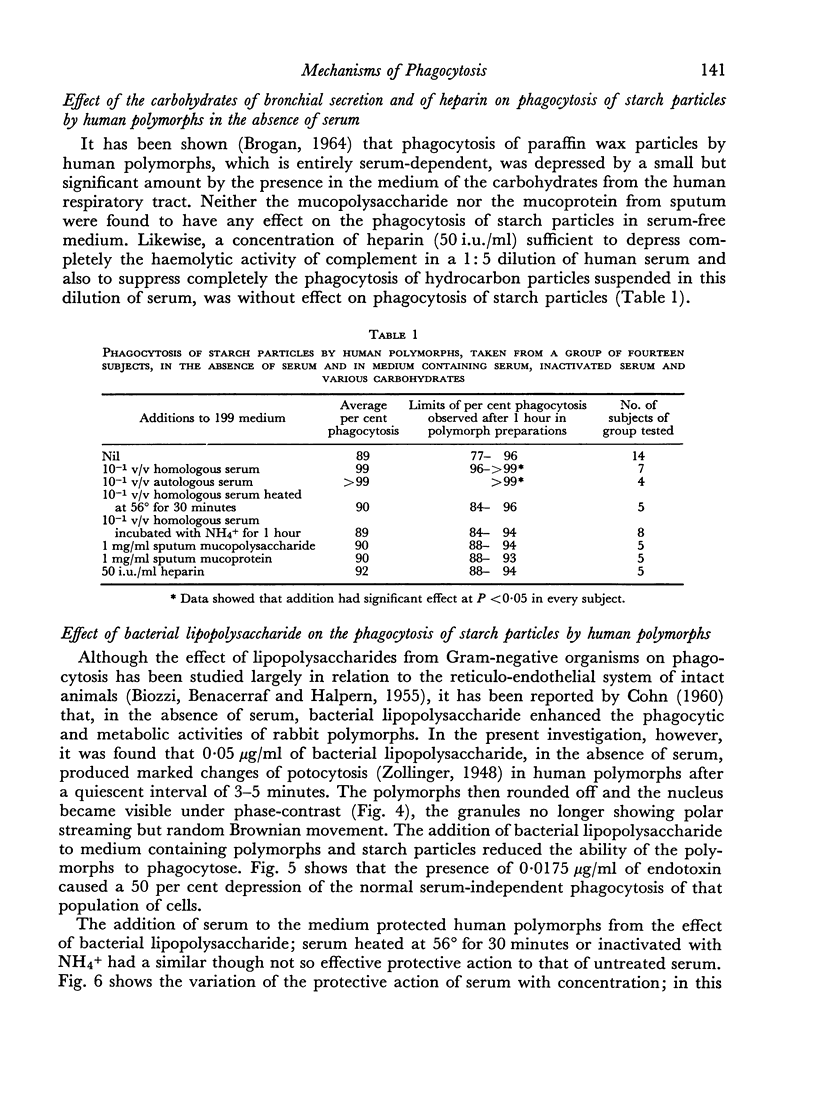
Ingestion of starch particles by human polymorphs in the absence of serum was unaffected by media at the extremes of physiological pH and at tonicities between 205 and 348 m-osmoles/l and by the presence in media of neutral and acid mucopolysaccharides.
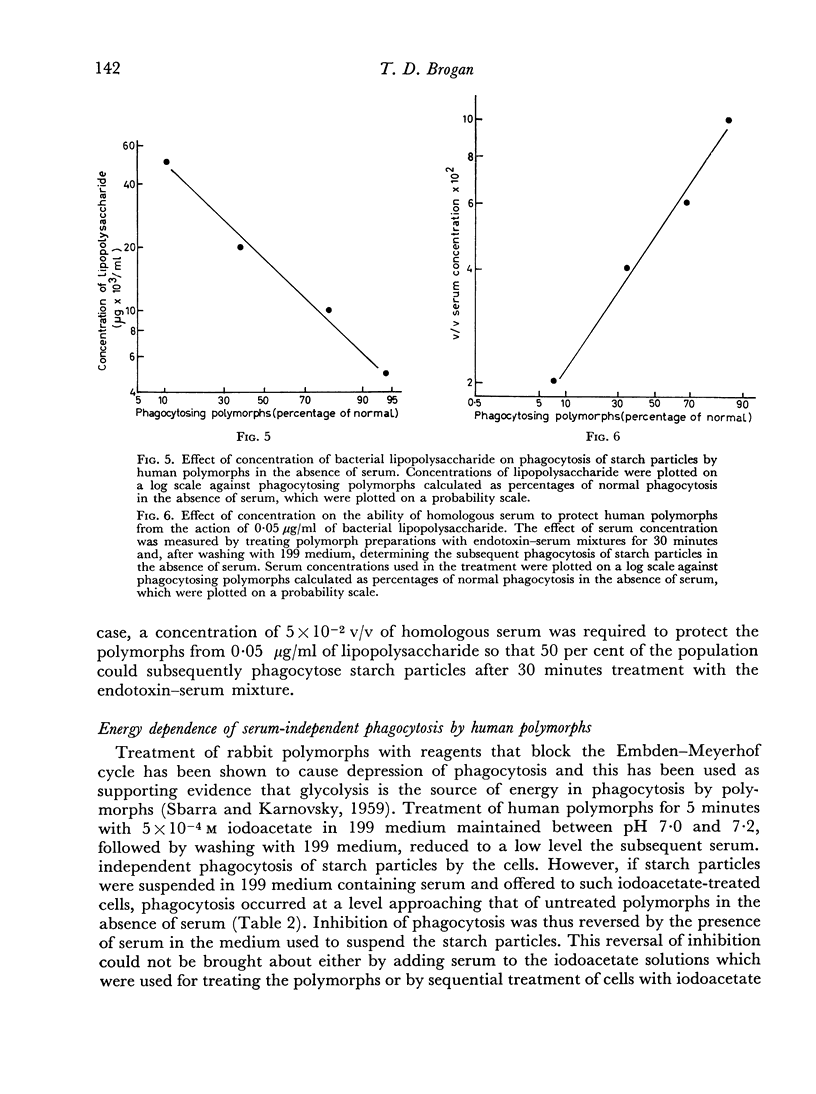
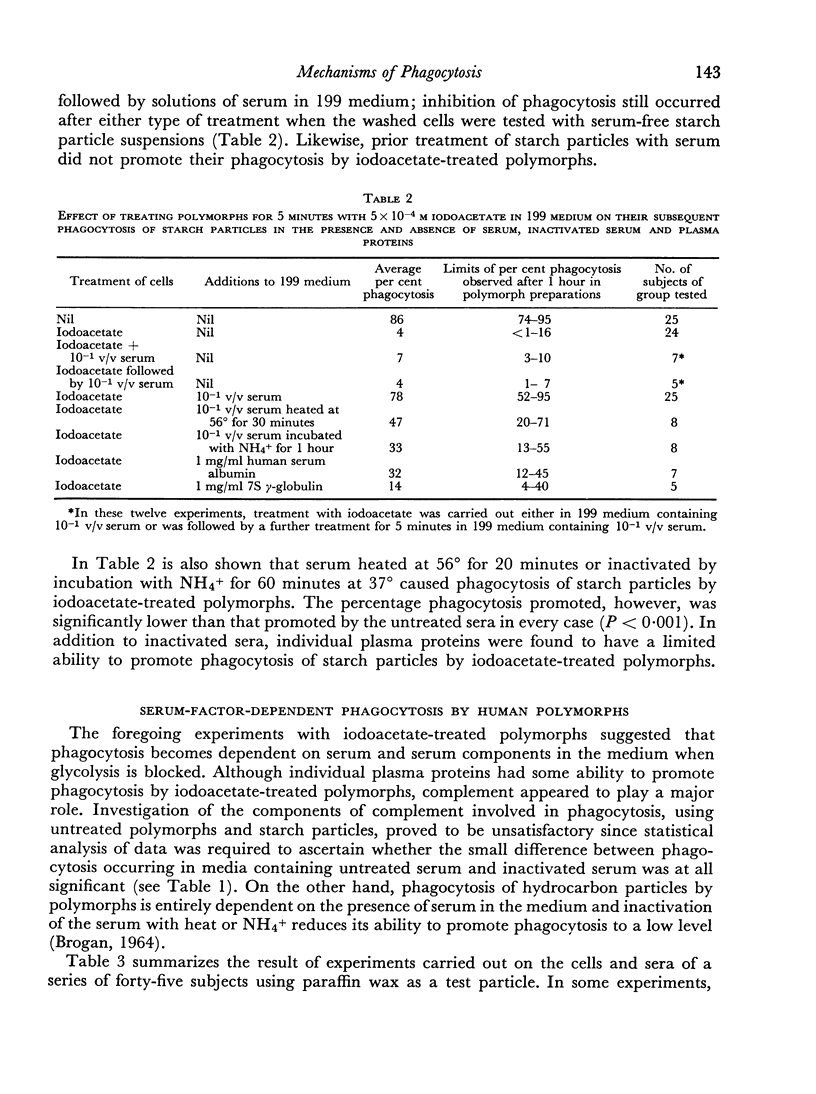
Phagocytosis of starch particles was reversibly inhibited by media of high tonicity and irreversibly inhibited by low concentrations of bacterial lipopolysaccharide, which had a lethal effect on the cells. Serum was unable to promote phagocytosis by polymorphs in media of high tonicity but both untreated serum and inactivated serum promoted phagocytosis in media containing endotoxin, probably by neutralizing the action of the lipopolysaccharide.
Phagocytosis of starch particles by human polymorphs in the absence of serum was inhibited by prior treatment of the cells with iodoacetate, suggesting that serum-independent phagocytosis relies on glycolytic energy. Ingestion of starch particles by polymorphs, treated with iodoacetate, was largely contingent on the presence of serum in the suspending medium, but inactivated serum and individual plasma proteins also had a limited ability to promote phagocytosis. These results suggested that cell glycolysis is not the principal source of energy in the serum-dependent mechanism of phagocytosis.
Using hydrocarbon test particles, it was shown that combinations of either of the heat-labile components of complement (C′1 or C′2) with the C′4 component were active in promoting phagocytosis. Evidence was also presented that the C′3 component had some activity and another serum factor, which was only present in the heat-inactivated sera of some individuals, also had a limited ability to promote phagocytosis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. The effect of Salm. typhi and its endotoxin on the phagocytic activity of the reticuloendothelial system in mice. Br J Exp Pathol. 1955 Jun;36(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- BROGAN T. D. EFFECT OF COMPLEMENT AND OF THE CARBOHYDRATE COMPONENTS OF SPUTUM ON PHAGOCYTOSIS BY HUMAN POLYMORPHONUCLEAR LEUCOCYTES. Immunology. 1964 Nov;7:626–638. [PMC free article] [PubMed] [Google Scholar]
- Brogan T. D. Deposition of human polymorphonuclear leucocytes on glass. Nature. 1965 Jul 17;207(994):316–317. doi: 10.1038/207316b0. [DOI] [PubMed] [Google Scholar]
- CHERNEW I., BRAUDE A. I. Depression of phagocytosis by solutes in concentrations found in the kidney and urine. J Clin Invest. 1962 Oct;41:1945–1953. doi: 10.1172/JCI104652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. Relation of cell metabolism to infection with rickettsial and bacterial agents. Bacteriol Rev. 1960 Mar;24(1):96–105. doi: 10.1128/br.24.1.96-105.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES R. R., SPENCER H., WAKELIN P. O. A CASE OF HUMAN PROTOTHECOSIS. Trans R Soc Trop Med Hyg. 1964 Sep;58:448–451. doi: 10.1016/0035-9203(64)90094-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLINGBECK J., HAYNES N. B., LAMMING G. E. INFLUENCE OF ISOLATED COMPONENTS OF THE UTERINE SECRETION ON PHAGOCYTOSIS. Nature. 1963 Jul 20;199:255–256. doi: 10.1038/199255a0. [DOI] [PubMed] [Google Scholar]
- RUDBACH J. A., JOHNSON A. G. RESTORATION OF ENDOTOXIN ACTIVITY FOLLOWING ALTERATION BY PLASMA. Nature. 1964 May 23;202:811–812. doi: 10.1038/202811a0. [DOI] [PubMed] [Google Scholar]
- RYTEL M. W., STOLLERMAN G. H. THE OPSONIC EFFECT OF COMPLEMENT ON THE PHAGOCYTOSIS OF GAMMA-GLOBULIN-COATED BENTONITE PARTICLES. J Immunol. 1963 Apr;90:607–611. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BAUMSTARK J. S. Effect of osmolarity on phagocytosis. J Bacteriol. 1963 Feb;85:306–313. doi: 10.1128/jb.85.2.306-313.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. I. The effects of endotoxin, endotoxin tolerance, and cortisone on the release of acid hydrolases from a granular fraction of rabbit liver. J Exp Med. 1962 Oct 1;116:433–450. doi: 10.1084/jem.116.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLLINGER H. U. Cytologic studies with the phase Microscope; the formation of blisters on cells in suspension, photocytosis, with observations on the nature of the cellular membrane. Am J Pathol. 1948 May;24(3):545–567. [PMC free article] [PubMed] [Google Scholar]